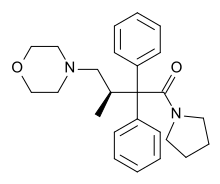
Levomoramide is the inactive isomer of the opioid analgesic dextromoramide, invented by the chemist Paul Janssen in 1956. Unlike dextromoramide, which is a potent analgesic with high abuse potential, levomoramide is virtually without activity.[2][3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.024.658 |
| Chemical and physical data | |
| Formula | C25H32N2O2 |
| Molar mass | 392.543 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
"Resolution reveals that the analgetic activity in this case resides almost entirely in the (+) isomer."[4]
"In the α-CH3 series, one of the optical isomers of each enantiomorphic pair is about twice as active as the racemic mixture; the other isomer is devoid of significant analgesic activity."[5]
However, despite being inactive, levomoramide is scheduled by UN Single Convention on Narcotic Drugs.
References
edit- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Janssen PA, Janseen JC (August 1956). "A new series of potent analgesics". Journal of the American Chemical Society. 78 (15): 3862. Bibcode:1956JAChS..78.3862J. doi:10.1021/ja01596a087.
- ^ Janssen PA, Jageneau AH (September 1957). "A new series of potent analgesics". Journal of Pharmacy and Pharmacology. 9 (1): 381–400. doi:10.1111/j.2042-7158.1957.tb12290.x. S2CID 58956931.
- ^ Lednicer D (1982). Central Analgetics. Wiley. p. 194. ISBN 0-471-08314-3.
- ^ Janssen PA (1960). Synthetic Analgesics Part 1: Diphenylpropylamines. Pergamon Press. p. 143.