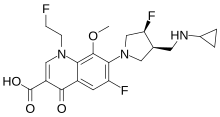
Lascufloxacin (trade name Lasvic) is an fluoroquinolone antibiotic drug for the treatment of bacterial infections. It has been approved since 2019 in Japan[1] to treat community-acquired pneumonia, otorhinolaryngological infections, and respiratory tract infections.[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Lasvic |
| Other names | KRP-AM1977 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H24F3N3O4 |
| Molar mass | 439.435 g·mol−1 |
| 3D model (JSmol) | |
| |
It has activity against various Gram-positive bacteria including Streptococcus pneumoniae[3] and Streptococcus anginosus.[4]
References
edit- ^ "Kyorin Pharmaceutical Receives Marketing Approval for Oral Quinolone Antibacterial Agent Lasvic Tablets 75 mg" (PDF) (Press release). Kyorin Pharmaceutical Co. September 20, 2019. Retrieved July 3, 2021.
- ^ "Lascufloxacin - Kyorin Pharmaceutical". Adis Insight. Retrieved July 3, 2021.
- ^ Murata M, Kosai K, Yamauchi S, Sasaki D, Kaku N, Uno N, Morinaga Y, Hasegawa H, Miyazaki T, Izumikawa K, Mukae H, Yanagihara K (April 2018). "In Vitro Activity of Lascufloxacin against Streptococcus pneumoniae with Mutations in the Quinolone Resistance-Determining Regions". Antimicrob Agents Chemother. 62 (4). doi:10.1128/AAC.01971-17. PMC 5913943. PMID 29439959.
- ^ Yamagishi Y, Matsukawa Y, Suematsu H, Mikamo H (December 2018). "In vitro activity of lascufloxacin, a novel fluoroquinolone antibacterial agent, against various clinical isolates of anaerobes and Streptococcus anginosus group". Anaerobe. 54: 61–64. doi:10.1016/j.anaerobe.2018.08.002. PMID 30114441. S2CID 52015090.