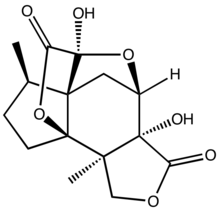
Jiadifenolide is a sesquiterpenoid natural product with neurotrophic activity, found in Illicium jiadifengpi. Its biological activity and congested polycyclic structure have made it a popular target for total synthesis.
| |
| Names | |
|---|---|
| IUPAC name
(1R,2R,6R,7R,9S,10R,11R)-6,9-Dihydroxy-2,11-dimethyl-4,8,14-trioxapentacyclo[7.4.2.17,10.01,10.02,6]hexadecane-5,15-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C15H18O7 | |
| Molar mass | 310.302 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isolation and bioactivity
editThe seco-prezizaane-type sesquiterpenoid jiadifenolide was isolated in 2009 from the fruit of the flowering plant Illicium jiadifengpi.[1] Chemical synthesis enabled preliminary assessment of its in vitro activity in promoting neurite outgrowth.[2]
Chemical synthesis
editJiadifenolide has been the subject of synthetic study in several academic labs.[3][4]
The first total synthesis, reported in 2009, employed an asymmetric Robinson annulation and a translactonization reaction to construct the core of the molecule.[5] Two further syntheses, a chiral-pool approach from (+)-pulegone[6] and a racemic synthesis relying on a samarium diiodide–mediated reductive cyclization,[7] were reported almost simultaneously in 2014. A second chiral-pool synthesis (from (+)-citronellal) reported in 2015 shortened the synthetic sequence, with a double Michael addition as the key transformation of eight steps, allowing the synthesis of gram-scale quantities of jiadifenolide.[8]
References
edit- ^ Kubo, Miwa; Okada, Chihiro; Huang, Jian-Mei; Harada, Kenichi; Hioki, Hideaki; Fukuyama, Yoshiyasu (19 November 2009). "Novel Pentacyclic seco-Prezizaane-Type Sesquiterpenoids with Neurotrophic Properties from Illicium jiadifengpi". Organic Letters. 11 (22): 5190–5193. doi:10.1021/ol9021029. PMID 19873982.
- ^ Trzoss, Lynnie; Xu, Jing; Lacoske, Michelle H.; Mobley, William C.; Theodorakis, Emmanuel A. (10 May 2013). "Illicium Sesquiterpenes: Divergent Synthetic Strategy and Neurotrophic Activity Studies". Chemistry: A European Journal. 19 (20): 6398–6408. doi:10.1002/chem.201300198. PMC 3875175. PMID 23526661.
- ^ Downer-Riley, Nadale K.; Jackson, Yvette A. (2012). "Highlight syntheses". Annual Reports on the Progress of Chemistry, Section B. 108: 147. doi:10.1039/c2oc90006h.
- ^ "(−)-Jiadifenolide". Chemistry World. Royal Society of Chemistry. Retrieved 29 July 2015.
- ^ Xu, Jing; Trzoss, Lynnie; Chang, Weng K.; Theodorakis, Emmanuel A. (11 April 2011). "Enantioselective Total Synthesis of (−)-Jiadifenolide". Angewandte Chemie International Edition. 50 (16): 3672–3676. doi:10.1002/anie.201100313. PMC 3159889. PMID 21400650.
- ^ Siler, David A.; Mighion, Jeffrey D.; Sorensen, Erik J. (19 May 2014). "An Enantiospecific Synthesis of Jiadifenolide". Angewandte Chemie International Edition. 53 (21): 5332–5335. doi:10.1002/anie.201402335. PMC 4153357. PMID 24757120.
- ^ Paterson, Ian; Xuan, Mengyang; Dalby, Stephen M. (7 July 2014). "Total Synthesis of Jiadifenolide". Angewandte Chemie International Edition. 53 (28): 7286–7289. doi:10.1002/anie.201404224. PMC 4320761. PMID 24861364.
- ^ Lu, Hai-Hua; Martinez, Michael D.; Shenvi, Ryan A. (15 June 2015). "An eight-step gram-scale synthesis of (−)-jiadifenolide". Nature Chemistry. 7 (7): 604–607. Bibcode:2015NatCh...7..604L. doi:10.1038/nchem.2283. PMID 26100810. S2CID 10715433.