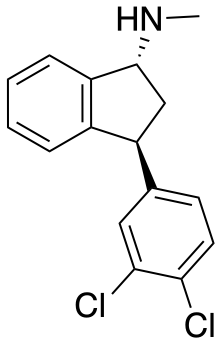
Indatraline hydrochloride (Lu 19-005) is an antidepressive agent and non-selective monoamine transporter inhibitor that blocks the reuptake of dopamine, norepinephrine, and serotonin with similar efficacy to cocaine.[1] This compound may be used to treat cocaine addictions as its effects have a slower onset and a longer duration than those of cocaine.[2] Lu 19-005 has been shown to block the action of methamphetamine and MDMA in laboratory experiments.[3]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H15Cl2N |
| Molar mass | 292.20 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Methylation
editIndatraline is N-alkylated at the amino group, making it possible to slow the onset of action, so that it is not until N-demethylation occurs that the molecules become active. N-methylindatraline has a longer duration than indatraline because norindatraline is inactive, whereas demethylating N-methylindatraline does not terminate the actions of the parent compound.
Effects of N-dimethylindatraline start about 20–30 minutes after administration; it takes a longer time for this chemical to absorb into the body than cocaine.[4]
Synthesis
editTwo main routes have been reported. The first route was reported by Bøgesø and co-workers.[5]
The other has been adapted to scale-up:[6]
Another method involves the contraction of a dihydronaphthalene (6–6 fused system) to form the 6–5 indane skeleton.[7]
Routes based on 1-indanone-type intermediates are not as simple as a direct reduction of an imine or oxime. The undesirable cis diastereomers are formed instead of the desirable trans isomers. This adds an extra step to the synthetic route. First, the ketones are reduced to mostly cis alcohols. Second, the cis alcohols are converted to the corresponding mesylates, conserving stereochemistry. Third, the mesylates can then be reacted, e.g. with, N-methylbenzylamine, causing a Walden inversion (SN2). Finally, the removal of the benzyl affords the product as a racemic mixture.
See also
editReferences
edit- ^ Cho YS, Yen CN, Shim JS, Kang DH, Kang SW, Liu JO, et al. (October 2016). "Antidepressant indatraline induces autophagy and inhibits restenosis via suppression of mTOR/S6 kinase signaling pathway". Scientific Reports. 6 (1): 34655. Bibcode:2016NatSR...634655C. doi:10.1038/srep34655. PMC 5046148. PMID 27694974.
- ^ Negus SS, Brandt MR, Mello NK (October 1999). "Effects of the long-acting monoamine reuptake inhibitor indatraline on cocaine self-administration in rhesus monkeys". The Journal of Pharmacology and Experimental Therapeutics. 291 (1): 60–69. PMID 10490887.
- ^ Rothman RB, Partilla JS, Baumann MH, Dersch CM, Carroll FI, Rice KC (March 2000). "Neurochemical neutralization of methamphetamine with high-affinity nonselective inhibitors of biogenic amine transporters: a pharmacological strategy for treating stimulant abuse". Synapse. 35 (3): 222–227. doi:10.1002/(SICI)1098-2396(20000301)35:3<222::AID-SYN7>3.0.CO;2-K. PMID 10657029. S2CID 16190813.
- ^ Gardner EL, Liu X, Paredes W, Giordano A, Spector J, Lepore M, et al. (October 2006). "A slow-onset, long-duration indanamine monoamine reuptake inhibitor as a potential maintenance pharmacotherapy for psychostimulant abuse: effects in laboratory rat models relating to addiction". Neuropharmacology. 51 (5): 993–1003. doi:10.1016/j.neuropharm.2006.06.009. PMID 16901516. S2CID 20465584.
- ^ Bøgesø KP, Christensen AV, Hyttel J, Liljefors T (December 1985). "3-Phenyl-1-indanamines. Potential antidepressant activity and potent inhibition of dopamine, norepinephrine, and serotonin uptake". Journal of Medicinal Chemistry. 28 (12): 1817–28. doi:10.1021/jm00150a012. PMID 2999402.
- ^ Froimowitz M, Wu KM, Moussa A, Haidar RM, Jurayj J, George C, et al. (December 2000). "Slow-onset, long-duration 3-(3',4'-dichlorophenyl)-1-indanamine monoamine reuptake blockers as potential medications to treat cocaine abuse". Journal of Medicinal Chemistry. 43 (26): 4981–92. doi:10.1021/jm000201d. PMID 11150168.
- ^ Silva LF, Siqueira FA, Pedrozo EC, Vieira FY, Doriguetto AC (April 2007). "Iodine(III)-promoted ring contraction of 1,2-dihydronaphthalenes: a diastereoselective total synthesis of (±)-indatraline". Organic Letters. 9 (8): 1433–6. doi:10.1021/ol070027o. PMID 17371034.