A hemangioma or haemangioma is a usually benign vascular tumor derived from blood vessel cell types. The most common form, seen in infants, is an infantile hemangioma, known colloquially as a "strawberry mark", most commonly presenting on the skin at birth or in the first weeks of life. A hemangioma can occur anywhere on the body, but most commonly appears on the face, scalp, chest or back. They tend to grow for up to a year before gradually shrinking as the child gets older. A hemangioma may need to be treated if it interferes with vision or breathing or is likely to cause long-term disfigurement. In rare cases internal hemangiomas can cause or contribute to other medical problems. They usually disappear by 10 years of age.[1] The first line treatment option is beta blockers, which are highly effective in the majority of cases. Hemangiomas present at birth are called congenital hemangiomas, while those that form later in life are called infantile hemangiomas.[2]
| Hemangioma | |
|---|---|
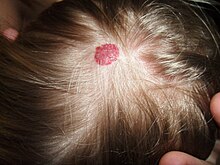 | |
| Hemangioma on the scalp | |
| Specialty | Oncology |
Types
editHemangiomas are benign (noncancerous) vascular tumors, and many different types occur. The correct terminology for these hemangioma types is constantly being updated by the International Society for the Study of Vascular Anomalies (ISSVA).[3] The most common are infantile hemangiomas, and congenital hemangiomas.
Infantile hemangiomas
editInfantile hemangiomas are the most common benign tumor found in children. They are made up of blood vessels, often called strawberry marks, and are more common in girls than in boys. Babies that are born early are more likely to have a hemangioma.[4] They usually appear on the skin of infants in the days or weeks after birth. They tend to grow quickly for up to a year. Most then shrink or involute without further problem, however some can ulcerate and form scabs which can be painful.[5] Depending on their location and size, they may also be disfiguring.
Rarely, they may be related to disorders of the central nervous system or spine. They may also occur in the internal organs of the body, such as the liver, airway or brain.[6]
The color of the hemangioma depends on how deep it is in the skin: superficial (near the skin's surface) hemangiomas tend to be bright red; deep (furthest from the skin's surface) hemangiomas are often blue or purple; mixed hemangiomas may have colors of both superficial and deep.[7]
Congenital hemangiomas
editCongenital hemangiomas are present on the skin at birth, unlike infantile hemangiomas, which appear later. They are fully formed at birth, meaning that they do not grow after a child is born, as infantile hemangiomas do. They are less common than infantile hemangiomas. Congenital hemangiomas can be coloured from pink to blue.
Congenital hemangiomas are classified according to whether they shrink and go away, or do not shrink, and do not go away, or partially shrink. Those that shrink are known as rapidly involuting congenital hemangiomas (RICH) and go away quickly. Those that do not shrink, and remain are known as noninvoluting congenital hemangiomas (NICH). Others that partially shrink are known as partially involuting congenital hemangiomas (PICH).[8][9]
Other types
editOther types of hemangioma include cavernous hemangiomas such as cavernous hemangioma of the liver.
Cavernous liver hemangioma
editA cavernous liver hemangioma or hepatic hemangioma is a benign tumour of the liver composed of hepatic endothelial cells. It is the most common liver tumour, and is usually asymptomatic and diagnosed incidentally on radiological imaging. Liver hemangiomas are thought to be congenital in origin.[10] Several subtypes exist, including the giant hepatic hemangioma, which can cause significant complications.
Drug-induced hemangioma
editDrug-induced hemangiomas are reported side-effects for some drugs in nonclinical toxicology animal models, studying carcinogenesis. For example, hemangiomas of the mesenteric lymph node were increased significantly at 700 mg/kg/day of Empagliflozin in male rats, or approximately 42 times the exposure from a 25 mg clinical dose.[11] It is inferred from nonclinical animal studies that some drugs can also produce hemangiomas in humans, and careful dosing during therapeutic drug design can ensure their safe use.
Diagnosis
editDiagnosis is usually clinical. Paediatric dermatologists are experts in diagnosing and treating hemangiomas. Depending on the location of the hemangioma, tests such as MRIs or ultrasounds can be done to see how far the hemangioma goes under the skin and whether it affects any internal organs.[12]
Treatment
editHemangiomas usually fade gradually over time, and many do not require treatment. However, hemangiomas that may be disfiguring or that are located at sites that can cause impairment (eyelids, airway) require early treatment intervention, typically with beta blockers. Management options may include:[13] A lot of treatment is based on case to case of each patient, with every case being different.[14]
- Oral beta blockers such as propranolol or atenolol have been used since 2008 and are the first-line treatment of hemangiomas. Beta blockers have repeatedly been shown to be effective and safe in treating hemangiomas that cause complications.[15] Beta blockers work via multiple mechanisms including narrowing the hemangioma's blood vessels, stopping them from proliferating and bringing forward their natural cell death. These correspond with hemangiomas fading and shrinking.[16] Approximately 97% of hemangiomas respond to propanolol, with patients under 2 months old showing the greatest improvement.[17]
- Topical beta blockers such as timolol. They are most helpful for thin superficial hemangiomas.[18] These should not be used in conjunction with oral beta blockers given systemic absorption of topical timolol is known to occur[19][20]
- Corticosteroids
- Laser surgery
- Physiotherapy for muscular skeletal pain management.[21]
References
edit- ^ Hemangioma: Types, causes & treatments. Cleveland Clinic. (n.d.). Retrieved December 7, 2022, from https://my.clevelandclinic.org/health/diseases/23365-hemangioma
- ^ Kita, Ashley E., and Jennifer L. Long. “Hemangioma.” Ear, nose, & throat journal 95.1 (2016): 19–20. Web.
- ^ "ISSVA Classification of Vascular Anomalies International Society for the Study of Vascular Anomalies" (PDF). Retrieved 11 August 2018.
- ^ Infantile hemangioma. Infantile Hemangioma | Johns Hopkins Medicine. (2022, July 15). Retrieved December 7, 2022, from https://www.hopkinsmedicine.org/health/conditions-and-diseases/infantile-hemangioma
- ^ Chang LC, Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Nopper AJ, Frieden IJ; Hemangioma Investigator Group. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008 Aug;122(2):360-7. doi: 10.1542/peds.2007-2767.
- ^ Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999 Jul 15;341(3):173-81.
- ^ "Infantile Hemangiomas". Retrieved 11 August 2018.
- ^ Mulliken JB, Bischoff J, Kozakewich HP. Multifocal rapidly involuting congenital hemangioma: a link to chorangioma. Am J Med Genet A. 2007;143A(24):3038-3046.
- ^ Funk T, Lim Y, Kulungowski AM, et al. Symptomatic Congenital Hemangioma and Congenital Hemangiomatosis Associated With a Somatic Activating Mutation in GNA11. JAMA Dermatol. 2016;152(9):1015-1020.
- ^ Baron R. 'Liver: Masses Part I: detection and characterization' Archived 2012-10-29 at the Wayback Machine. The Radiology Assistant 2006
- ^ Prescribing Information: JARDIANCE® (empagliflozin). Section 13.1
- ^ "Hemangioma".
- ^ "Hemangioma - Diagnosis and treatment - Mayo Clinic". www.mayoclinic.org. Retrieved 2021-03-19.
- ^ Kynion, Richard. “Hemangiomas.” Pediatrics in review 38.4 (2017): 191–193. Web.
- ^ "What is the role of propranolol in the treatment of infantile hemangiomas?".
- ^ Nguyen, Harrison P.; Pickrell, Brent B.; Wright, Teresa S. (May 2014). "Beta-Blockers as Therapy for Infantile Hemangiomas". Seminars in Plastic Surgery. 28 (2): 87–90. doi:10.1055/s-0034-1376259. ISSN 1535-2188. PMC 4078206. PMID 25045334.
- ^ Zhang, Ling; Wu, Hai-Wei; Yuan, Weien; Zheng, Jia-Wei (2017-05-08). "Propranolol therapy for infantile hemangioma: our experience". Drug Design, Development and Therapy. 11: 1401–1408. doi:10.2147/DDDT.S134808. ISSN 1177-8881. PMC 5428756. PMID 28507428.
- ^ Topical Timolol Maleate Treatment of Infantile Hemangiomas. Püttgen K, Lucky A, Adams D, Pope E, McCuaig C, Powell J, Feigenbaum D, Savva Y, Baselga E, Holland K, Drolet B, Siegel D, Morel KD, Garzon MC, Mathes E, Lauren C, Nopper A, Horii K, Newell B, Song W, Frieden I; Hemangioma Investigator Group. Pediatrics. 2016 Sep;138(3):e20160355. doi: 10.1542/peds.2016-0355. Epub 2016 Aug 15. PMID 27527799
- ^ Systemic timolol exposure following topical application to infantile hemangiomas. Drolet BA, Boakye-Agyeman F, Harper B, Holland K, Lewandowski A, Stefanko N, Melloni C; Pediatric Trials Network Steering Committee (See Acknowledgments for a listing of committee members.). J Am Acad Dermatol. 2020 Mar;82(3):733-736. doi: 10.1016/j.jaad.2019.02.029. Epub 2019 Feb 18. PMID 30790601 .
- ^ Ge, Jing; Zheng, Jiawei; Zhang, Ling; Yuan, Weien; Zhao, Haiguang (2016-01-28). "Oral propranolol combined with topical timolol for compound infantile hemangiomas: a retrospective study". Scientific Reports. 6: 19765. Bibcode:2016NatSR...619765G. doi:10.1038/srep19765. ISSN 2045-2322. PMC 4730155. PMID 26819072.
- ^ Chu, Eric Chun-Pu (23 June 2022). "Conservative Management of Low Back Pain Related to an Unresectable Aggressive Sacral Hemangioma: A Case Report". American Journal of Case Reports. 23 (e936984): e936984. doi:10.12659/AJCR.936984. PMC 9238883. PMID 35733328. Retrieved 28 June 2022.