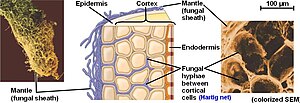
The Hartig net is the network of inward-growing hyphae, that extends into the plant host root, penetrating between plant cells in the root epidermis and cortex in ectomycorrhizal symbiosis.[1][2] This network is the internal component of fungal morphology in ectomycorrhizal symbiotic structures formed with host plant roots, in addition to a hyphal mantle or sheath on the root surface, and extramatrical mycelium extending from the mantle into the surrounding soil. The Hartig net is the site of mutualistic resource exchange between the fungus and the host plant. Essential nutrients for plant growth are acquired from the soil by exploration and foraging of the extramatrical mycelium, then transported through the hyphal network across the mantle and into the Hartig net, where they are released by the fungi into the root apoplastic space for uptake by the plant. The hyphae in the Hartig net acquire sugars from the plant root, which are transported to the external mycelium to provide a carbon source to sustain fungal growth.[3]
Structure and Development
editThe Hartig net is a lattice-like network of hyphae that grow into the plant root from the hyphal mantle at the plant root surface. The hyphae of ectomycorrhizal fungi do not penetrate the plant cells, but occupy the apoplastic space between cells in the root. This network extends between the epidermal cells near the root surface, and may also extend between cells in the root cortex.[2][4] The hyphae in the Hartig net formed by some ECM fungi are described as having transfer-cell like structures, with highly folded membranes that increase surface area and facilitate secretion and uptake of resources exchanged in the mutualistic symbiosis.[5]
The initiation of hyphal growth into the intercellular space between roots often begins between 2–4 days following the establishment of the hyphal mantle in contact with the root surface.[6][7] The initial development of the Hartig net likely involves a regulated decrease of plant defense responses, thus allowing fungal infection. Studies carried out with the model ectomycorrhizal fungus Laccaria bicolor have shown that the fungus secretes a small effector protein (MISSP7) that may regulate plant defense mechanisms by controlling plant response to phytohormones.[8] Unlike some plant root pathogenic fungi, ectomycorrhizal fungi are largely unable to produce many plant cell-wall-degrading enzymes, but increased pectin modification enzymes released by Laccaria bicolor during fungal infection and Hartig net development indicate that pectin degradation may function to loosen the adhesion between neighboring plant cells and allow room for hyphal growth between cells[9][10]
This Hartig net structure is common among ectomycorrhizal fungi, although the depth and thickness of the hyphal network can vary considerably depending on the host species. Fungi associating with plants in the Pinaceae form a robust Hartig net that penetrates between cells deep into the root cortex, while the Hartig net formation in ectomycorrhizal symbioses with many angiosperms may not extend beyond the root epidermis.[11] It has also been demonstrated that the depth and development of the Hartig net can vary among different fungi, even among isolates of the same species. Interestingly, an experiment using two isolates of Paxillus involutus, one of which only developed a loose mantle at the root surface and no developed Hartig net in poplar roots, showed that plant nitrate uptake was still improved by the symbiosis regardless of the presence of internal hyphal structure.[12] As an additional caveat some fungal species such as Tuber melanosporum can form arbutoid mycorrhizae, involving some intracellular penetration into plant root cells by fungal hyphae in addition to developing a shallow Hartig-net-like structure between epidermal cells.[13]
Function
editThe Hartig net supplies the plant root with chemical elements required for plant growth, such as nitrogen and phosphorus,[14] potassium,[15][16] and micronutrients[17] in addition to water supplied to the roots through hyphal transport.[18] Essential nutrients acquired from surrounding soil by the extramatrical mycelium are transported into the hyphae in the Hartig net, where they are released into the apoplastic space for direct uptake directly by plant root cells.[3][19]
In exchange for the nutrients provided by the fungal partner, the plant provides a portion of its photosynthetically fixed carbon to the fungal partner as sugars. Sugars are released into the apoplastic space and made available for uptake by Hartig net hyphae. Although sucrose was long considered to be an important form of carbon provided by the plant to the fungus, many ectomycorrhizal fungi lack sucrose uptake transporters. Therefore, the fungal symbiont may depend on plant production of invertases to degrade sucrose into useable monosaccharides for fungal uptake.[20][21] In the Hartig net of Amanita muscaria within poplar roots, expression of important fungal enzymes for trehalose biosynthesis was higher than in the extrametrical mycelium, indicating that trehalose production may function as a carbohydrate sink, increasing fungal demand of plant photosynthesized carbon compounds through the symbiotic exchange.[22] The plant regulatory mechanisms that influence the nutrient supply by the Hartig net are not fully understood, but it is thought that upregulation of plant defense mechanisms in response to decreased nitrogen transport by ECM fungi, rather than reductions in carbon allocation to ECM roots, suggesting that the regulation of symbiotic resource exchange for ECM symbiosis is not a simple reciprocal response.[20]
In addition to the exchange of essential nutrients, the Hartig net may play an important role in plant strategies for tolerance of abiotic stressors, such as regulating bioaccumulation of metals[23][24] or mediating plant stress responses to salinity.[12]
Name
editThe Hartig net is named after Theodor Hartig,[25][26] a 19th-century German forest biologist and botanist. He reported research in 1842 on the anatomy of the interface between ectomycorrhizal fungi and tree roots.
See also
editReferences
edit- ^ Smith, Sally, Read, David (2002). "Chapter 6: Structure and Development of Ectomycorrhizal roots". Mycorrhizal Symbiosis. IV–V: 163–232. doi:10.1016/B978-012652840-4/50007-3.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Brundrett, Mark C.; Tedersoo, Leho (December 2018). "Evolutionary history of mycorrhizal symbioses and global host plant diversity". New Phytologist. 220 (4): 1108–1115. doi:10.1111/nph.14976. ISSN 0028-646X. PMID 29355963.
- ^ a b Becquer, Adeline; Guerro-Galan, Carmen (2019). "Chapter Three: The Ectomychorrhizal contribution to tree nutrition". Advances in Botanical Research. 89: 77–126. doi:10.1016/bs.abr.2018.11.003. S2CID 92840690.
- ^ Nylund, Jan‐Erik (December 1980). "Symplastic Continuity During Hartig Net Formation in Norway Spruce Ectomycorrhizae". New Phytologist. 86 (4): 373–378. doi:10.1111/j.1469-8137.1980.tb01678.x. ISSN 0028-646X.
- ^ Kottke, I.; Oberwinkler, F. (March 1987). "The cellular structure of the Hartig net: coenocytic and transfer cell‐like organization". Nordic Journal of Botany. 7 (1): 85–95. doi:10.1111/j.1756-1051.1987.tb00919.x. ISSN 0107-055X.
- ^ Le Quéré, Antoine; Wright, Derek P.; Söderström, Bengt; Tunlid, Anders; Johansson, Tomas (July 2005). "Global Patterns of Gene Regulation Associated with the Development of Ectomycorrhiza Between Birch ( Betula pendula Roth.) and Paxillus involutus (Batsch) Fr". Molecular Plant-Microbe Interactions. 18 (7): 659–673. doi:10.1094/MPMI-18-0659. ISSN 0894-0282. PMID 16042012.
- ^ Horan, D. P.; Chilvers, G. A.; Lapeyrie, F. F. (August 1988). "Time sequence of the infection process eucalypt ectomycorrhizas". New Phytologist. 109 (4): 451–458. doi:10.1111/j.1469-8137.1988.tb03720.x. ISSN 0028-646X.
- ^ Daguerre, Yohann; Basso, Veronica; Hartmann-Wittulski, Sebastian; Schellenberger, Romain; Meyer, Laura; Bailly, Justine; Kohler, Annegret; Plett, Jonathan M.; Martin, Francis; Veneault-Fourrey, Claire (2020-11-23). "The mutualism effector MiSSP7 of Laccaria bicolor alters the interactions between the poplar JAZ6 protein and its associated proteins". Scientific Reports. 10 (1): 20362. Bibcode:2020NatSR..1020362D. doi:10.1038/s41598-020-76832-6. ISSN 2045-2322. PMC 7683724. PMID 33230111. S2CID 227152666.
- ^ Su, Chao (April 2023). "Pectin modifications at the symbiotic interface". New Phytologist. 238 (1): 25–32. doi:10.1111/nph.18705. ISSN 0028-646X. PMID 36565041. S2CID 255115317.
- ^ Chowdhury, Jamil; Kemppainen, Minna; Delhomme, Nicolas; Shutava, Iryna; Zhou, Jingjing; Takahashi, Junko; Pardo, Alejandro G.; Lundberg‐Felten, Judith (October 2022). "Laccaria bicolor pectin methylesterases are involved in ectomycorrhiza development with Populus tremula × Populus tremuloides". New Phytologist. 236 (2): 639–655. doi:10.1111/nph.18358. ISSN 0028-646X. PMC 9796311. PMID 35794841.
- ^ Brundrett, Mark C.; Tedersoo, Leho (2020-09-01). "Resolving the mycorrhizal status of important northern hemisphere trees". Plant and Soil. 454 (1): 3–34. Bibcode:2020PlSoi.454....3B. doi:10.1007/s11104-020-04627-9. ISSN 1573-5036. S2CID 220931730.
- ^ a b Sa, Gang; Yao, Jun; Deng, Chen; Liu, Jian; Zhang, Yinan; Zhu, Zhimei; Zhang, Yuhong; Ma, Xujun; Zhao, Rui; Lin, Shanzhi; Lu, Cunfu; Polle, Andrea; Chen, Shaoliang (June 2019). "Amelioration of nitrate uptake under salt stress by ectomycorrhiza with and without a Hartig net". New Phytologist. 222 (4): 1951–1964. doi:10.1111/nph.15740. ISSN 0028-646X. PMC 6594093. PMID 30756398.
- ^ Ori, Francesca; Leonardi, Marco; Faccio, Antonella; Sillo, Fabiano; Iotti, Mirco; Pacioni, Giovanni; Balestrini, Raffaella (2020-11-01). "Synthesis and ultrastructural observation of arbutoid mycorrhizae of black truffles (Tuber melanosporum and T. aestivum)". Mycorrhiza. 30 (6): 715–723. Bibcode:2020Mycor..30..715O. doi:10.1007/s00572-020-00985-5. ISSN 1432-1890. PMC 7591440. PMID 33079241.
- ^ Nehls, Uwe; Plassard, Claude (2018-06-11). "Nitrogen and phosphate metabolism in ectomycorrhizas". New Phytologist. 220 (4): 1047–1058. doi:10.1111/nph.15257. ISSN 0028-646X. PMID 29888395.
- ^ Maria del Carmen Guerrero-Galan, Gabriella Houdinet, Amandine Delteil, Kevin Garcia, Sabine Zimmermann. Unravelling nutrient exchange in ectomycorrhizal symbiosis contributing to plant potassium nutrition. International Conference Saclay Plant Sciences (SPS) 2018, Jul 2018, Paris, France. ⟨hal-01843727⟩
- ^ Guerrero‐Galán, C., Delteil, A., Garcia, K., Houdinet, G., Conéjéro, G., Gaillard, I., Sentenac, H. and Zimmermann, S.D. (2018), Plant potassium nutrition in ectomycorrhizal symbiosis: properties and roles of the three fungal TOK potassium channels in Hebeloma cylindrosporum. Environ Microbiol, 20: 1873-1887. doi:10.1111/1462-2920.14122
- ^ Ruytinx, Joske; Kafle, Arjun; Usman, Muhammad; Coninx, Laura; Zimmermann, Sabine D.; Garcia, Kevin (2020-03-01). "Micronutrient transport in mycorrhizal symbiosis; zinc steals the show". Fungal Biology Reviews. 34 (1): 1–9. doi:10.1016/j.fbr.2019.09.001. hdl:1942/31110. ISSN 1749-4613. S2CID 208574971.
- ^ Heinonsalo, Jussi; Juurola, Eija; Linden, Aki; Pumpanen, Jukka (2015-01-01). "Ectomycorrhizal fungi affect Scots pine photosynthesis through nitrogen and water economy, not only through increased carbon demand". Environmental and Experimental Botany. 109: 103–112. doi:10.1016/j.envexpbot.2014.08.008. ISSN 0098-8472.
- ^ Roy, R., Reinders, A., Ward, J. M., & McDonald, T. R. (2020). Understanding transport processes in lichen, Azolla-cyanobacteria, ectomycorrhiza, endomycorrhiza, and rhizobia-legume symbiotic interactions. F1000Research, 9, F1000 Faculty Rev-39. https://doi.org/10.12688/f1000research.19740.1
- ^ a b Stuart, Emiko K.; Plett, Krista L. (2020). "Digging Deeper: In Search of the Mechanisms of Carbon and Nitrogen Exchange in Ectomycorrhizal Symbioses". Frontiers in Plant Science. 10: 1658. doi:10.3389/fpls.2019.01658. ISSN 1664-462X. PMC 6971170. PMID 31993064.
- ^ Salzer, P.; Hager, A. (December 1991). "Sucrose Utilization of the Ectomycorrhizal Fungi Amanita muscaria and Hebeloma crustuliniforme Depends on the Cell Wall‐bound Invertase Activity of their Host Picea abies". Botanica Acta. 104 (6): 439–445. doi:10.1111/j.1438-8677.1991.tb00256.x. ISSN 0932-8629.
- ^ López, Mónica Fajardo; Männer, Philipp; Willmann, Anita; Hampp, Rüdiger; Nehls, Uwe (April 2007). "Increased trehalose biosynthesis in Hartig net hyphae of ectomycorrhizas". New Phytologist. 174 (2): 389–398. doi:10.1111/j.1469-8137.2007.01983.x. ISSN 0028-646X. PMID 17388901.
- ^ Shi, W, Zhang, Y, Chen, S, Polle, A, Rennenberg, H, Luo, Z‐B. Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ. 2019; 42: 1087– 1103. https://doi.org/10.1111/pce.13471
- ^ Frey, B.; Zierold, K.; Brunner, I. (November 2000). "Extracellular complexation of Cd in the Hartig net and cytosolic Zn sequestration in the fungal mantle of Picea abies – Hebeloma crustuliniforme ectomycorrhizas". Plant, Cell & Environment. 23 (11): 1257–1265. doi:10.1046/j.1365-3040.2000.00637.x. ISSN 0140-7791.
- ^ Money, Nicholas P (2011). Mushroom. Oxford: Oxford University Press. p. 71.
- ^ Maser, C; Claridge, A W; Trappe, J M (2008). Trees, Truffles, and Beasts: How Forests Function. New Brunswick: Rutgers University Press. p. 54.