| ||
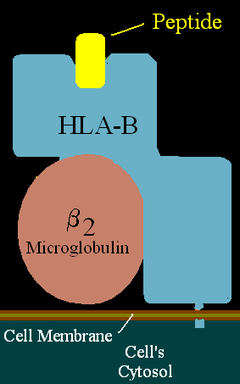
| HLA-B (alpha)-β2MG with bound peptide | ||
major histocompatibility complex (human), class I, B39
| ||
| Alleles | B*3901, 3902, 3903, . . | |
| Structure (See HLA-B) | ||
| Shared data | ||
| Locus | chr.6 6p21.31 | |
HLA-B39 (B39) is an HLA-B serotype. The serotype identifies the more common HLA-B*39 gene products.[1]
B39 is a split antigen of the broad antigen B16, and is a sister type of B38. B39 is most commonly found on the west pacific rim, in Japan and highest frequency in the new world. In Europe it is found in Scandinavia and Northern Russia.
Serotype
edit| B*39 | B39 | B16 | B38 | Sample | |
| allele | % | % | % | size (N) | |
| *3901 | 95 | 476 | |||
| *3902 | 87 | 96 | |||
| *3903 | 77 | 20 | |||
| *3905 | 82 | 245 | |||
| *3906 | 94 | 508 | |||
| *3908 | 48 | 57 | |||
| *3909 | 97 | 13 | |||
| *3910 | 82 | 127 | |||
| *3911 | 69 | 16 | |||
| Alleles link-out to IMGT/HLA Databease at EBI | |||||
The serology for the most common B39 alleles, B*3901 and B*3906 is good, but some allele products are not well detected. Given the differential involvement of these alleles in disease testing should involve high resolution typing.
| freq | ||
| ref. | Population | (%) |
| [3] | Venezuela Perja Mountain Bari | 23.9 |
| Mexico Mixtec Oaxaca | 8.8 | |
| USA Arizona Pima | 7.3 | |
| USA South Texas Hispanics | 5.6 | |
| Mexico Zaptotec Oaxaca | 4.5 | |
| New Mexico Canoncito Navajo | 3.7 | |
| Oman | 2.5 | |
| PNG Wanigela | 2.3 | |
| Tunisia Tunis | 2.3 | |
| North American Natives | 1.9 | |
| USA Hispanic | 1.7 | |
| Brazil Belo Horizonte | 1.6 | |
| South Dakota Lakota Sioux | 1.5 | |
| Cuban White | 1.4 | |
| Azores S. Maria & S. Miguel | 1.3 | |
| Australia New South Wales | 1.1 | |
| Finland | 1.1 | |
| Portugal North | 1.1 | |
| Portugal Centre | 1.0 | |
| Spain Eastern Andalusia Gipsy | 1.0 | |
| Brazil Terena | 0.9 | |
| Ireland Northern | 0.9 | |
| Azores Terceira Island | 0.8 | |
| USA Caucasian pop2 | 0.8 | |
| Brazil | 0.7 | |
| USA Philadelphia Caucasians | 0.7 | |
| India Andhra Pradesh Golla | 0.5 | |
| Thailand | 0.4 |
Alleles
edit| freq | ||
| ref. | Population | (%) |
| [3] | Taiwan Saisiat | 54.9 |
| [3] | Taiwan Tsou | 24.5 |
| [3] | South Dakota Lakota Sioux | 22.5 |
| [3] | Taiwan Taroko | 21.8 |
| [3] | Taiwan Atayal | 19.8 |
| [3] | PNG Wanigela | 16.7 |
| [3] | Japan Ainu Hokkaido | 16.0 |
| [3] | Taiwan Bunun | 14.9 |
| [3] | New Mexico Canoncito Navajo | 14.6 |
| [3] | Taiwan Thao | 13.3 |
| [3] | Taiwan Rukai | 13.0 |
| [3] | Taiwan Ami | 10.2 |
| [3] | USA Hawaii Okinawa | 7.7 |
| [3] | Papua New Guinea Wosera | 7.0 |
| [3] | Papua New Guinea Madang | 6.4 |
| [3] | Mexico Mixtec Oaxaca | 5.9 |
| [3] | Taiwan Puyuma | 5.0 |
| [3] | Taiwan Paiwan | 4.9 |
| [3] | New Caledonia | 4.8 |
| [3] | Japan Central | 4.4 |
| [3] | American Samoa | 4.0 |
| [3] | USA North American Natives | 4.0 |
| [3] | Taiwan Hakka | 3.6 |
| [3] | Indig. Australian Cape York Peninsula | 3.5 |
| [3] | Japan (5) | 3.5 |
| [3] | Philippines Ivatan | 3.0 |
| [3] | Brazil | 2.9 |
| [3] | China Guangxi Maonan | 2.8 |
| [3] | Georgia Tbilisi Georgians | 2.8 |
| [3] | China Yunnan Nu | 2.6 |
| [3] | Thailand | 2.5 |
| [3] | Azores Terceira Island | 2.4 |
| [3] | Singapore Chinese | 2.4 |
| [3] | Romanian | 2.3 |
| [3] | China Yunnan Lisu | 2.2 |
| [3] | Indig. Australian Yuendumu | 2.1 |
| [3] | China Guangzhou | 2.0 |
| [3] | Croatia | 2.0 |
| [3] | France South East | 1.9 |
| [3] | Georgia Svaneti Svans | 1.9 |
| [3] | PNG Karimui Plateau | 1.9 |
| [3] | Azores Central Islands | 1.8 |
| [3] | Spain Eastern Andalusia | 1.8 |
| [3] | Uganda Kampala | 1.6 |
| [3] | Mexico Zaptotec Oaxaca | 1.5 |
| [3] | Singapore Thai | 1.5 |
| [3] | China Guangzhou Han | 1.4 |
| [3] | China Qinghai Hui | 1.4 |
| [3] | Czech Republic | 1.4 |
| [3] | Madeira | 1.4 |
| [3] | Indig. Australian Kimberly | 1.3 |
| [3] | Finland | 1.1 |
Disease
editB39 is suggested as a factor in Takayasu's arteritis and gallstones in Mexico.[4] Osteoarticular complications of brucellosis appear to be associated with B39.[5] An association with spondylarthropathies[6][7] and psoriatic arthritis[8][9] was observed in several studies. Psoriatic arthritis appears to be linked to MICA-A9 which tightly linked to HLA-B39.[10][11] B39 also appears to be involved in the Fishers syndrome variant of Guillain–Barré syndrome.[12]
B39 appears to be protective against cardiomyopathy in Chaga's disease indicating a possible selective factor in its rise in the New World.[13] Chaga's disease is caused by a trypanosome carried by a blood sucking insect found in tropical, palm growing regions.
Southern California now reports cases of Chaga's disease from contaminated transfusions and may be already a habitat for the vector.[14]
In Takayasu's arteritis
editTakayasu's arteritis appears to have a link to B39.[15][16] The association with B*3902 increases risk of pulmonary infarction, ischemic heart disease, aortic regurgitation, systemic hypertension, renal artery stenosis, cerebrovascular disease, and visual disturbance.[17] B*3906, common in indigenous Mesoamericans has been found associated with the same disease.[18]
| freq | ||
| ref. | Population | (%) |
| [3] | Mexico Mixe Oaxaca | 38.7 |
| Mexico Zaptotec Oaxaca | 13.4 | |
| Mexico Mixtec Oaxaca | 5.9 | |
| Mexico Mestizos | 1.2 | |
| Japan pop5 | 0.9 | |
| Cuban White | 0.7 | |
| Israel Ashk. and Non Ashk. Jews | 0.5 | |
| Japan Central | 0.5 | |
| Senegal Niokholo Mandenka | 0.5 | |
| B*3903 | ||
|---|---|---|
| PNG New Britain Rabaul | 13.2 | |
| Brazil Terena | 11.2 | |
| Argentina Toba Rosario | 5.2 | |
| Brazil | 0.7 | |
| Finland | 0.6 | |
| Kenya Luo | 0.6 | |
| North American Natives | 0.5 | |
| B*3904 | ||
| Argentina Toba Rosario | 1.2 | |
| Georgia Svaneti Svans | 0.6 | |
| Jordan Amman | 0.3 | |
| Shijiazhuang Tianjian Han | 0.1 | |
| Japan Central | 0.1 | |
| Romanian | 0.1 | |
| B*3905 | ||
| Venezuela Perija Yucpa | 36.1 | |
| Mexico Zaptotec Oaxaca | 12.7 | |
| Mexico Mixtec Oaxaca | 9.8 | |
| Mexico Mestizos | 4.9 | |
| Arizona Pima | 4.5 | |
| Mexico Guadalajara Mestizos | 2.4 | |
| Argentina Toba Rosario | 2.3 | |
| USA Hispanic | 2.1 | |
| Mexico Mixe Oaxaca | 1.9 | |
| Cuban White | 1.4 | |
| Mexico Chihuahua Tarahumara | 1.1 | |
| USA North American Natives | 0.5 | |
| Shijiazhuang Tianjian Han | 0.2 | |
| B*3907 | ||
| Shijiazhuang Tianjian Han | 0.6 | |
| Singapore Thai | 0.5 | |
| China South Han | 0.2 | |
| B*3908 | ||
| Mexico Zaptotec Oaxaca | 2.2 | |
| Mexico Mestizos | 1.2 | |
| Mexico Mixtec Oaxaca | 1.0 | |
| Brazil | 0.7 | |
| USA Hispanic | 0.6 | |
| B*3909 | ||
| Venezuela Perija Yucpa | 34.9 | |
| Thailand pop3 | 3.1 | |
| Brazil Terena | 1.7 | |
| China South Han | 1.4 | |
| Argentina Toba Rosario | 1.2 | |
| China Qinghai Hui | 0.9 | |
| B*3910 | ||
| Sudanese | 2.5 | |
| Senegal Niokholo Mandenka | 2.1 | |
| South African Natal Zulu | 1.5 | |
| Cameroon Beti | 1.4 | |
| Spain Eastern Andalusia | 1.2 | |
| Kenya Luo | 1.1 | |
| Israel Arab Druse | 1.0 | |
| Kenya Nandi | 1.0 | |
| Guinea Bissau | 0.8 | |
| Kenya | 0.7 | |
| Mali Bandiagara | 0.7 | |
| Morocco Nador Metalsa Class I | 0.7 | |
| Cameroon Bamileke | 0.6 | |
| Cameroon Yaounde | 0.5 | |
| Israel Ashk. and Non Ashk. Jews | 0.5 | |
| Saudi Arabia Guraiat and Hail | 0.5 | |
References
edit- ^ Marsh, S. G.; Albert, E. D.; Bodmer, W. F.; Bontrop, R. E.; Dupont, B.; Erlich, H. A.; Fernández-Viña, M.; Geraghty, D. E.; Holdsworth, R.; Hurley, C. K.; Lau, M.; Lee, K. W.; Mach, B.; Maiers, M.; Mayr, W. R.; Müller, C. R.; Parham, P.; Petersdorf, E. W.; Sasazuki, T.; Strominger, J. L.; Svejgaard, A.; Terasaki, P. I.; Tiercy, J. M.; Trowsdale, J. (2010). "Nomenclature for factors of the HLA system, 2010". Tissue Antigens. 75 (4): 291–455. doi:10.1111/j.1399-0039.2010.01466.x. PMC 2848993. PMID 20356336.
- ^ derived from IMGT/HLA
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb Middleton D, Menchaca L, Rood H, Komerofsky R (2003). "New allele frequency database". Tissue Antigens. 61 (5): 403–7. doi:10.1034/j.1399-0039.2003.00062.x. PMID 12753660.
- ^ Méndez-Sánchez N, King-Martínez AC, Ramos MH, Pichardo-Bahena R, Uribe M (November 2004). "The Amerindian's genes in the Mexican population are associated with development of gallstone disease". Am. J. Gastroenterol. 99 (11): 2166–70. PMID 15554998.
- ^ Bravo MJ, Colmenero Jde D, Alonso A, Caballero A (May 2003). "HLA-B*39 allele confers susceptibility to osteoarticular complications in human brucellosis". J. Rheumatol. 30 (5): 1051–3. PMID 12734905.
- ^ Sobao Y, Tsuchiya N, Takiguchi M, Tokunaga K (January 1999). "Overlapping peptide-binding specificities of HLA-B27 and B39: evidence for a role of peptide supermotif in the pathogenesis of spondylarthropathies". Arthritis Rheum. 42 (1): 175–81. doi:10.1002/1529-0131(199901)42:1<175::AID-ANR21>3.0.CO;2-7. PMID 9920028.
- ^ Alvarez I, López de Castro JA (July 2000). "HLA-B27 and immunogenetics of spondyloarthropathies". Curr Opin Rheumatol. 12 (4): 248–53. doi:10.1097/00002281-200007000-00003. PMID 10910175.
- ^ Eastmond CJ (May 1994). "Psoriatic arthritis. Genetics and HLA antigens". Baillière's Clinical Rheumatology. 8 (2): 263–76. doi:10.1016/S0950-3579(94)80018-9. PMID 8076387.
- ^ Gladman DD, Farewell VT (June 1995). "The role of HLA antigens as indicators of disease progression in psoriatic arthritis. Multivariate relative risk model". Arthritis Rheum. 38 (6): 845–50. doi:10.1002/art.1780380619. PMID 7779129.
- ^ Bolognesi E, Dalfonso S, Rolando V, Fasano ME, Praticò L, Momigliano-Richiardi P (October 2001). "MICA and MICB microsatellite alleles in HLA extended haplotypes". Eur. J. Immunogenet. 28 (5): 523–30. doi:10.1046/j.0960-7420.2001.00250.x. PMID 11881819.
- ^ González S, Martínez-Borra J, López-Vázquez A, García-Fernández S, Torre-Alonso JC, López-Larrea C (May 2002). "MICA rather than MICB, TNFA, or HLA-DRB1 is associated with susceptibility to psoriatic arthritis". J. Rheumatol. 29 (5): 973–8. PMID 12022360. Archived from the original on 2008-12-04. Retrieved 2008-08-03.
- ^ Yuki N, Sato S, Tsuji S, Ogawa K, Miyatake T (June 1993). "Human leukocyte antigens in Fisher's syndrome". Ann. Neurol. 33 (6): 655–7. doi:10.1002/ana.410330617. PMID 8498847.
- ^ Cruz-Robles D, Reyes PA, Monteón-Padilla VM, Ortiz-Muñiz AR, Vargas-Alarcón G (January 2004). "MHC class I and class II genes in Mexican patients with Chagas disease". Hum. Immunol. 65 (1): 60–5. doi:10.1016/j.humimm.2003.10.008. PMID 14700597.
- ^ "Chagas Disease Represents New Challenge for Blood Supply Safety".
- ^ Kimura A, Kitamura H, Date Y, Numano F (August 1996). "Comprehensive analysis of HLA genes in Takayasu arteritis in Japan". Int. J. Cardiol. 54 Suppl: S61–9. doi:10.1016/s0167-5273(96)88774-2. PMID 9119528.
- ^ Yoshida M, Kimura A, Katsuragi K, Numano F, Sasazuki T (August 1993). "DNA typing of HLA-B gene in Takayasu's arteritis". Tissue Antigens. 42 (2): 87–90. doi:10.1111/j.1399-0039.1993.tb02242.x. PMID 7903491.
- ^ Kitamura H, Kobayashi Y, Kimura A, Numano F (October 1998). "Association of clinical manifestations with HLA-B alleles in Takayasu arteritis". Int. J. Cardiol. 66 Suppl 1: S121–6. doi:10.1016/S0167-5273(98)00159-4. PMID 9951811.
- ^ Rodríguez-Reyna TS, Zúñiga-Ramos J, Salgado N, et al. (October 1998). "Intron 2 and exon 3 sequences may be involved in the susceptibility to develop Takayasu arteritis". Int. J. Cardiol. 66 Suppl 1: S135–8, discussion S139. doi:10.1016/S0167-5273(98)00161-2. PMID 9951813.