G-protein coupled receptor 31 also known as 12-(S)-HETE receptor is a protein that in humans is encoded by the GPR31 gene. The human gene is located on chromosome 6q27 and encodes a G-protein coupled receptor protein composed of 319 amino acids.[5][6]
| GPR31 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | GPR31, 12-HETER, HETER, HETER1, G protein-coupled receptor 31 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 602043; MGI: 1354372; HomoloGene: 48337; GeneCards: GPR31; OMA:GPR31 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Function
editThe GPR31 receptor shares a close amino acid sequence similarity with the oxoeicosanoid receptor 1, a G-protein coupled receptor encoded by the GPR170 gene.[7][8][9]
Ligand binding and activation
editThe oxoeicosanoid receptor 1 is the receptor for a group of arachidonic acid metabolites produced by 5-lipoxygenase, such as 5-Hydroxyicosatetraenoic acid (5-HETE), 5-oxoicosanoic acid (5-oxo-ETE), and other members of this family, which are potent bioactive cell stimuli. In contrast, the GPR31 receptor binds to a different arachidonic acid metabolite, 12-hydroxyeicosatetraenoic acid (12-HETE), synthesized by 12-lipoxygenase. This conclusion is supported by studies that cloned the receptor from the PC-3 prostate cancer cell line. The cloned receptor, when expressed in other cell types, bound 12-HETE with high affinity (Kd = 5 nM) and mediated the effects of low concentrations of the S but not R stereoisomer of 12-HETE.[9]
In a [35S]GTPγS binding assay, which estimates a receptor's binding affinity by measuring its stimulation of [35S]GTPγS binding, 12(S)-HETE activated GPR31 with an EC50 (effective concentration causing a 50% of maximal [35S]GTPγS binding) of less than 0.3 nM. In comparison, the EC50 was 42 nM for 15(S)-HETE, 390 nM for 5(S)-HETE, and undetectable for 12(R)-HETE.[10]
It is currently unknown whether GPR31 interacts with structural analogs of 12(S)-HETE, such as 12-oxo-ETE (a metabolite of 12(S)-HETE), various 5,12-diHETEs including LTB4, or other bioactive metabolites like the hepoxilins. Further research is required to determine whether GPR31 exclusively binds and mediates the effects of 12(S)-HETE or, like the oxoeicosanoid receptor 1, interacts with a broader family of analogs.
Signaling pathways
editLike the oxoeicosanoid receptor, GPR31 activates the MEK-ERK1/2 signaling pathway, but unlike oxoeicosanoid receptor 1, it does not cause an increase in cytosolic Ca2+ concentration. It also activates NFκB.[9] GPR31 exhibits stereospecificity and other properties expected of a true G-protein coupled receptor (GPCR).
Additional receptors activated by 12(S)-HETE
edit12(S)-HETE also: a) binds to and activates the leukotriene B4 receptor-2 (BLT2), a GPCR for the 5-lipoxygenase-derived metabolite LTB4;[9][11] b) binds to, but inhibits, the GPCR for prostaglandin H2 and thromboxane A2, two arachidonic acid metabolites;[12] c) binds with high affinity to a 50 kilodalton (kDa) subunit of a 650 kDa cytosolic and nuclear protein complex;[13] and d) binds with low affinity to and activates intracellular peroxisome proliferator-activated receptor gamma.[14]
Complications in determining GPR31 function
editThese alternate binding sites complicate the determination of 12(S)-HETE's reliance on GPR31 for cell activation and the overall function of GPR31. Studies utilizing GPR31 Gene knockout models will be crucial for understanding its role in vivo.
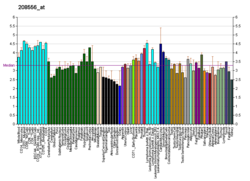
Tissue distribution
editGPR31 receptor mRNA is highly expressed in the PC-3 prostate cancer cell line and to a lesser extent the DU145 prostate cancer cell line and to human umbilical vein endothelial cells (HUVEC), human umbilical vein endothelial cells (HUVEC), human brain microvascular endothelial cells (HBMEC), and human pulmonary aortic endothelial cells (HPAC).[9] Its mRNA is also express but at rather low levels in several other human cell lines including: K562 cells (human myelogenous leukemia cells); Jurkat cells (T lymphocyte cells); Hut78 cells (T cell lymphoma cells), HEK 293 cells (primary embryonic kidney cells), MCF-7 cells (mammary adenocarcinoma cellss), and EJ cells (bladder carcinoma cells).[5][6]
Mice express an ortholog to human GPR31 in their circulating blood platelets.[15]
Clinical significance
editProstate cancer
editThe GPR31 receptor appears to mediate the responses of PC-3 prostate cancer cells to 12(S)-HETE in stimulating the MEK-ERK1/2 and NFκB pathways and therefore may contribute to the growth-promoting and metastasis-promoting actions that 12(S)-HETE is proposed to have in human prostate cancer.[16][17][18] However, LNCaP and PC3 human prostate cancer cells also express BLT2 receptors; in LNCaP cells, BLT2 receptors stimulate the expression of the growth- and metastasis-promoting androgen receptor;[19] in PC3 cells, BLT2 receptors stimulate the NF-κB pathway to inhibit the apoptosis induced by cell detachment from surfaces (i.e. Anoikis;[20] and, in BLT2-overexpressing PWR-1E non-malignant prostate cells, 12(S)-HETE diminished anoikis-associated apoptotic cell death.[20] Thus, the roles of 12(S)-HETE in human prostate cancer, if any, may involve its activation of either or both GPR31 and BLT2 receptors.
Other diseases
editThe many other actions of 12(S)-HETE (see 12-Hydroxyeicosatetraenoic acid) and any other ligands found to interact with this receptor will require studies similar those conducted on PC3 cells[10] and mesenteric arteries[15] to determine the extent to which they interact with BLT2, TXA2/PGH2, and PPARgamma receptors and thereby may contribute in part or whole to their activity. Clues implicating the GPR31, as opposed to the other receptors in the actions of 12(S)-HETE include findings that GPR31 receptors do not respond to 12(R)-HETE nor induce rises in cytosolic Ca2+ whereas the other receptors mediate one or both of these actions. These studies will be important because, in addition to prostate cancer, preliminary studies suggest that the GPR31 receptor is implicated in several other diseases such as malignant megakaryocytis (Acute megakaryoblastic leukemia), arthritis, Alzheimer's disease, progressive B-cell chronic lymphocytic leukemia, Diabetic neuropathy, and high grade astrocytoma.[10]
References
edit- ^ a b c GRCh38: Ensembl release 89: ENSG00000120436 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000071311 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b Zingoni A, Rocchi M, Storlazzi CT, Bernardini G, Santoni A, Napolitano M (June 1997). "Isolation and chromosomal localization of GPR31, a human gene encoding a putative G protein-coupled receptor". Genomics. 42 (3): 519–523. doi:10.1006/geno.1997.4754. hdl:11573/245592. PMID 9205127.
- ^ a b "Entrez Gene: GPR31 G protein-coupled receptor 31".
- ^ Hosoi T, Koguchi Y, Sugikawa E, Chikada A, Ogawa K, Tsuda N, et al. (August 2002). "Identification of a novel human eicosanoid receptor coupled to G(i/o)". The Journal of Biological Chemistry. 277 (35): 31459–31465. doi:10.1074/jbc.M203194200. PMID 12065583.
- ^ Jones CE, Holden S, Tenaillon L, Bhatia U, Seuwen K, Tranter P, et al. (March 2003). "Expression and characterization of a 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils". Molecular Pharmacology. 63 (3): 471–477. doi:10.1124/mol.63.3.471. PMID 12606753.
- ^ a b c d e Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly AK, et al. (September 2011). "Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid". The Journal of Biological Chemistry. 286 (39): 33832–33840. doi:10.1074/jbc.M110.216564. PMC 3190773. PMID 21712392.
- ^ a b c Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly AK, et al. (September 2011). "Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid". The Journal of Biological Chemistry. 286 (39): 33832–33840. doi:10.1074/jbc.M110.216564. PMC 3190773. PMID 21712392.
- ^ O'Flaherty JT, Cordes JF, Lee SL, Samuel M, Thomas MJ (December 1994). "Chemical and biological characterization of oxo-eicosatetraenoic acids". Biochimica et Biophysica Acta (BBA) - General Subjects. 1201 (3): 505–515. doi:10.1016/0304-4165(94)90083-3. PMID 7803484.
- ^ Fonlupt P, Croset M, Lagarde M (July 1991). "12-HETE inhibits the binding of PGH2/TXA2 receptor ligands in human platelets". Thrombosis Research. 63 (2): 239–248. doi:10.1016/0049-3848(91)90287-7. PMID 1837628.
- ^ Herbertsson H, Kühme T, Hammarström S (July 1999). "The 650-kDa 12(S)-hydroxyeicosatetraenoic acid binding complex: occurrence in human platelets, identification of hsp90 as a constituent, and binding properties of its 50-kDa subunit". Archives of Biochemistry and Biophysics. 367 (1): 33–38. doi:10.1006/abbi.1999.1233. PMID 10375396.
- ^ Li Q, Cheon YP, Kannan A, Shanker S, Bagchi IC, Bagchi MK (March 2004). "A novel pathway involving progesterone receptor, 12/15-lipoxygenase-derived eicosanoids, and peroxisome proliferator-activated receptor gamma regulates implantation in mice". The Journal of Biological Chemistry. 279 (12): 11570–11581. doi:10.1074/jbc.M311773200. PMID 14688261.
- ^ a b Siangjong L, Gauthier KM, Pfister SL, Smyth EM, Campbell WB (February 2013). "Endothelial 12(S)-HETE vasorelaxation is mediated by thromboxane receptor inhibition in mouse mesenteric arteries". American Journal of Physiology. Heart and Circulatory Physiology. 304 (3): H382–H392. doi:10.1152/ajpheart.00690.2012. PMC 3774504. PMID 23203967.
- ^ Nie D, Krishnamoorthy S, Jin R, Tang K, Chen Y, Qiao Y, et al. (July 2006). "Mechanisms regulating tumor angiogenesis by 12-lipoxygenase in prostate cancer cells". The Journal of Biological Chemistry. 281 (27): 18601–18609. doi:10.1074/jbc.M601887200. PMID 16638750.
- ^ Yang P, Cartwright CA, Li J, Wen S, Prokhorova IN, Shureiqi I, et al. (October 2012). "Arachidonic acid metabolism in human prostate cancer". International Journal of Oncology. 41 (4): 1495–1503. doi:10.3892/ijo.2012.1588. PMC 3982713. PMID 22895552.
- ^ Porro B, Songia P, Squellerio I, Tremoli E, Cavalca V (August 2014). "Analysis, physiological and clinical significance of 12-HETE: a neglected platelet-derived 12-lipoxygenase product". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 964: 26–40. doi:10.1016/j.jchromb.2014.03.015. PMID 24685839.
- ^ Lee JW, Kim GY, Kim JH (April 2012). "Androgen receptor is up-regulated by a BLT2-linked pathway to contribute to prostate cancer progression". Biochemical and Biophysical Research Communications. 420 (2): 428–433. doi:10.1016/j.bbrc.2012.03.012. PMID 22426480.
- ^ a b Lee JW, Kim JH (October 2013). "Activation of the leukotriene B4 receptor 2-reactive oxygen species (BLT2-ROS) cascade following detachment confers anoikis resistance in prostate cancer cells". The Journal of Biological Chemistry. 288 (42): 30054–30063. doi:10.1074/jbc.M113.481283. PMC 3798474. PMID 23986446.