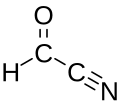
Formyl cyanide is a simple organic compound with the formula HCOCN and structure HC(=O)−C≡N. It is simultaneously a nitrile (R−C≡N) and an aldehyde (R−CH=O). Formyl cyanide is the simplest member of the acyl cyanide family. It is known to occur in space in the Sgr B2 molecular cloud.[1]
| |
| Names | |
|---|---|
| Preferred IUPAC name
Formyl cyanide | |
| Systematic IUPAC name
Methanoyl cyanide | |
| Other names
Cyanoformaldehyde
Glyoxylonitrile 2-oxo-acetonitrile oxo-acetonitrile | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2HNO | |
| Molar mass | 55.036 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Production
editFormyl cyanide was first made through methoxyacetonitrile flash vacuum pyrolysis at 600 °C. The same technique with cinnamyloxyacetonitrile[2] or allyloxyacetonitrile also generates formyl cyanide.[3][4]
In molecular clouds, formation of formyl cyanide is speculated to result from formaldehyde and the cyanide radical:[5]
- CH2O + CN• → HCOCN + H•
In Earth's atmosphere, the pollutant acrylonitrile reacts with hydroxyl radical forming formyl cyanide, hydroperoxyl and formaldehyde:[6]
- CH2=CHCN + HO• + 3⁄2 O2 → HOO• + HCOCN + CH2O
Reactions
editFormyl cyanide reacts rapidly with even trace quantities of water to form formic acid and hydrogen cyanide. In scrupulously dry conditions, the compound instead releases carbon monoxide, with a half-life exceeding 45 h.[2]
Related
editBy formally substituting the hydrogen atom, cyanoformyl chloride, ClC(O)CN, and cyanoformyl bromide, BrC(O)CN are obtained.[7]
References
edit- ^ Gronowski, Marcin; Eluszkiewicz, Piotr; Custer, Thomas Gage (12 April 2017). "Structure and Spectroscopy of C2HNO Isomers". The Journal of Physical Chemistry A. 121 (17): 3263–3273. Bibcode:2017JPCA..121.3263G. doi:10.1021/acs.jpca.6b12609. PMID 28402122.
- ^ a b Lewis-Bevan, Wyn; Gaston, Rick D.; Tyrrell, James; Stork, Wilmer D.; Salmon, Gary L. (March 1992). "Formyl cyanide: a stable species. Experimental and theoretical studies". Journal of the American Chemical Society. 114 (6): 1933–1938. doi:10.1021/ja00032a001.
- ^ Bogey, M.; Destombes, J.L.; Vallee, Y.; Ripoll, J.L. (May 1988). "Formyl cyanide: Efficient production from allyloxyacetonitrile and its millimeter-wave spectrum". Chemical Physics Letters. 146 (3–4): 227–229. Bibcode:1988CPL...146..227B. doi:10.1016/0009-2614(88)87435-9.
- ^ Bogey, M.; Demuynck, C.; Destombes, J.L.; Vallee, Y. (August 1995). "Millimeter-Wave Spectrum of Formyl Cyanide, HCOCN: Centrifugal Distortion and Hyperfine Structure Analysis". Journal of Molecular Spectroscopy. 172 (2): 344–351. Bibcode:1995JMoSp.172..344B. doi:10.1006/jmsp.1995.1183.
- ^ Remijan, Anthony J.; Hollis, J. M.; Lovas, F. J.; Stork, Wilmer D.; Jewell, P. R.; Meier, D. S. (10 March 2008). "Detection of Interstellar Cyanoformaldehyde (CNCHO)". The Astrophysical Journal. 675 (2): L85–L88. Bibcode:2008ApJ...675L..85R. doi:10.1086/533529. S2CID 19005362.
- ^ Grosjean, Daniel (December 1990). "Atmospheric Chemistry of Toxic Contaminants. 3. Unsaturated Aliphatics: Acrolein, Acrylonitrile, Maleic Anhydride". Journal of the Air & Waste Management Association. 40 (12): 1664–1669. Bibcode:1990JAWMA..40.1664G. doi:10.1080/10473289.1990.10466814.
- ^ Pasinszki, Tibor; Vass, Gábor; Klapstein, Dieter; Westwood, Nicholas P. C. (5 April 2012). "Generation, Spectroscopy, and Structure of Cyanoformyl Chloride and Cyanoformyl Bromide, XC(O)CN". The Journal of Physical Chemistry A. 116 (13): 3396–3403. Bibcode:2012JPCA..116.3396P. doi:10.1021/jp301528q. PMID 22409314.