The fluorenylmethoxycarbonyl protecting group (Fmoc) is a base-labile amine protecting group used in organic synthesis, particularly in peptide synthesis[1]. It is popular for its stability toward acids and hydrolysis and its selective removal by weak bases, such as piperidine, without affecting most other protecting groups or sensitive functional groups. Fmoc protection is especially advantageous in solid-phase peptide synthesis (SPPS), where its compatibility with other reagents and ease of removal streamline synthesis workflows. Upon deprotection, Fmoc yields a byproduct (Dibenzofulvene) that can be monitored by UV spectroscopy, allowing for efficient reaction tracking.[2]
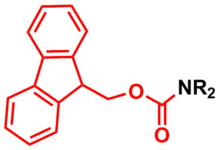
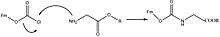
Protection & Formation
editFmoc-carbamate is frequently used as a protecting group for primary and secondery amines, where the Fmoc group can be introduced by reacting the amine with fluorenylmethyloxycarbonyl chloride (Fmoc-Cl), e.g.:[3]
The other common method for introducing the Fmoc group is through 9-fluorenylmethylsuccinimidyl carbonate (Fmoc-OSu), which may itself be obtained by the reaction of Fmoc-Cl with the dicyclohexylammonium salt of N-hydroxysuccinimide.[4]
Reacting with 9-fluorenylmethyloxycarbonyl azide (itself made by reacting Fmoc-Cl with sodium azide) in sodium bicarbonate and aqueous dioxane is also a method to install Fmoc group.[1] Because the fluorenyl group is highly fluorescent, certain UV-inactive compounds may be reacted to give the Fmoc derivatives, suitable for analysis by reversed phase HPLC. Analytical uses of Fmoc-Cl that do not use chromatography may be limited by the requirement that excess Fmoc-Cl be removed before an analysis of fluorescence.
Cleavage & Deprotection
editThe Fmoc group is rapidly removed by base. Piperidine is usually preferred for Fmoc group removal as it forms a stable adduct with the dibenzofulvene byproduct, preventing it from reacting with the substrate.[5][6]
Role in Peptide Synthesis
editThe use of Fmoc as a temporary protecting group for amine at the N-terminus in solid phase synthesis is very widespread for Fmoc/tBu approach, because its removal with piperidine does not disturb the acid-labile linker between the peptide and the resin.[7] A typical SPPS Fmoc deprotection is performed with a solution of 20% piperidine in N,N-dimethylformamide (DMF).[8]
- C13H9−CH2−OC(O)NHR + (CH2)5NH → (CH2)5NH+2 + [C13H8−CH2−OC(O)NHR]−
- [C13H8−CH2−OC(O)NHR]− → C13H8=CH2 + −OC(O)NHR
- −OC(O)NHR + (CH2)5NH+2 → HOC(O)NHR + (CH2)5NH
- HOC(O)NHR → CO2 + RNH2
- C13H8=CH2 + (CH2)5NH → C13H9−CH2N(CH2)5
Common deprotection cocktails for Fmoc during SPPS
edit- 20% piperidine in DMF (Fmoc group has an approximate half life of 6 seconds in this solution)[8]
- 5% piperazine, 1% DBU and 1% formic acid in DMF. This method avoids the use of strictly controlled piperidine.[9] No side product was observed for a peptide with 9 residues synthesized with this method.[10]
References
edit- ^ a b Carpino, Louis A.; Han, Grace Y. (November 1972). "9-Fluorenylmethoxycarbonyl amino-protecting group". The Journal of Organic Chemistry. 37 (22): 3404–3409. doi:10.1021/jo00795a005. ISSN 0022-3263.
- ^ Várady, László; Rajur, Shranabasava B; Nicewonger, Robert B; Guo, MaoJun; Ditto, Lori (2000-02-11). "Fast and quantitative high-performance liquid chromatography method for the determination of 9-fluorenylmethoxycarbonyl release from solid-phase synthesis resins". Journal of Chromatography A. 869 (1): 171–179. doi:10.1016/S0021-9673(99)00844-4. ISSN 0021-9673. PMID 10720236.
- ^ Yamada, Kazuhiko; Hashizume, Daisuke; Shimizu, Tadashi; Ohki, Shinobu; Yokoyama, Shigeyuki (2008). "A solid-state 17O NMR, X-ray, and quantum chemical study of N-α-Fmoc-protected amino acids". Journal of Molecular Structure. 888 (1–3): 187–196. doi:10.1016/j.molstruc.2007.11.059.
- ^ Paquet, A. (1982). "Introduction of 9-fluorenylmethyloxycarbonyl, trichloroethoxycarbonyl, and benzyloxycarbonyl amine protecting groups into O-unprotected hydroxyamino acids using succinimidyl carbonates". Canadian Journal of Chemistry. 60 (8): 976–980. doi:10.1139/v82-146.
- ^ Fields, Gregg B. (1995), Pennington, Michael W.; Dunn, Ben M. (eds.), "Methods for Removing the Finoc Group", Peptide Synthesis Protocols, Methods in Molecular Biology, vol. 35, Totowa, NJ: Humana Press, pp. 17–27, doi:10.1385/0-89603-273-6:17, ISBN 978-1-59259-522-8, PMID 7894598, retrieved 2021-10-15
- ^ Wellings, Donald A.; Atherton, Eric (1997). "[4] Standard Fmoc protocols". Solid-Phase Peptide Synthesis. Methods in Enzymology. Vol. 289. pp. 44–67. doi:10.1016/s0076-6879(97)89043-x. ISBN 9780121821906. PMID 9353717.
- ^ J. Jones, Amino Acid and Peptide Synthesis, 2nd edn., Oxford University Press, 2002
- ^ a b Wuts, P. G. M.; Greene, T.W. (2006). Greene's Protective Groups in Organic Synthesis. NY: J. Wiley. doi:10.1002/0470053488. ISBN 9780470053485.
- ^ Ralhan, Krittika; KrishnaKumar, V. Guru; Gupta, Sharad (8 December 2015). "Piperazine and DBU: a safer alternative for rapid and efficient Fmoc deprotection in solid phase peptide synthesis". RSC Advances. 5 (126): 104417–104425. Bibcode:2015RSCAd...5j4417R. doi:10.1039/C5RA23441G. ISSN 2046-2069.
- ^ Lam, Pak-Lun; Wu, Yue; Wong, Ka-Leung (30 March 2022). "Incorporation of Fmoc-Dab(Mtt)-OH during solid-phase peptide synthesis: a word of caution". Organic & Biomolecular Chemistry. 20 (13): 2601–2604. doi:10.1039/D2OB00070A. ISSN 1477-0539. PMID 35258068. S2CID 247175352.
External links
edit- Media related to Fmoc at Wikimedia Commons