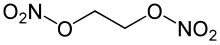
Ethylene glycol dinitrate, abbreviated EGDN and NGC, also known as Nitroglycol, is a colorless, oily, explosive liquid obtained by nitrating ethylene glycol. It is similar to nitroglycerine in both manufacture and properties, though it is more volatile and less viscous. Unlike nitroglycerine, the chemical has a perfect oxygen balance, meaning that its ideal exothermic decomposition would completely convert it to low energy carbon dioxide, water, and nitrogen gas, with no excess unreacted substances, without needing to react with anything else.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Ethane-1,2-diyl dinitrate | |
| Other names
Ethylene glycol dinitrate, Glycol dinitrate, Ethylene dinitrate, Ethylene nitrate, 1,2-Bis(nitrooxy)ethane, Nitroglycol (NGc), 1,2-Ethanediol dinitrate, Dinitroglycol, EGDN, Ethane-1,2-diyl dinitrate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.010.058 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H4N2O6 | |
| Molar mass | 152.1 g/mol |
| Appearance | Oily, colorless to light yellow liquid |
| Odor | odorless[1] |
| Density | 1.4918 g/cm3 |
| Melting point | −22.0 °C (−7.6 °F; 251.2 K) |
| Boiling point | 197.5 °C (387.5 °F; 470.6 K) |
| 5 g/l | |
| Vapor pressure | 0.05 mmHg (@ 20 °C)[1] |
| Explosive data | |
| Shock sensitivity | 0.02 kp m = 0.2 Nm |
| Friction sensitivity | 36 kp = 353 N pistil load no reaction |
| Detonation velocity | 8300 m/s[2][circular reference] |
| RE factor | 1.66 |
| Hazards | |
| GHS labelling: | |
   
| |
| NFPA 704 (fire diamond) | |
| Flash point | 215 °C; 419 °F; 488 K[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
C 0.2 ppm (1 mg/m3) [skin][1] |
REL (Recommended)
|
ST 0.1 mg/m3 [skin][1] |
IDLH (Immediate danger)
|
75 mg/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
History and production
editPure EGDN was first produced by the Belgian chemist Louis Henry (1834–1913) in 1870 by dropping a small amount of ethylene glycol into a mixture of nitric and sulfuric acids cooled to 0 °C.[3] The previous year, August Kekulé had produced EGDN by the nitration of ethylene, but this was actually contaminated with beta-nitroethyl nitrate.[4][5][6]
Other investigators preparing NGC before publication in 1926 of Rinkenbach's work included: Champion (1871), Neff (1899) & Wieland & Sakellarios (1920), Dautriche, Hough & Oehme.
The American chemist William Henry Rinkenbach (1894–1965) prepared EGDN by nitrating purified glycol obtained by fractioning the commercial product under pressure of 40mm Hg, and at a temperature of 120°. For this 20g of middle fraction of purified glycol was gradually added to mixture of 70g nitric acid and 130g sulfuric acid, maintaining the temperature at 23°. The resulting 49g of crude product was washed with 300ml of water to obtain 39.6g of purified product. The low yield so obtained could be improved by maintaining a lower temperature and using a different nitrating acid mixture.[5]
1) Direct Nitration of Glycol is carried out in exactly the same manner, with the same apparatus, and with the same mixed acids as nitration of glycerine.
In the test nitration of anhydrous glycol (100g) with 625g of mixed acid HNO
3 40% & H
2SO
4 60% at 10-12°, the yield was 222g and it dropped to 218g when the temp was raised to 29-30°.
When 500g of mixed acid HNO
3 50% & H
2SO
4 50% was used at 10-12°, the yield increased to 229g.
In commercial nitration, the yields obtained from 100 kg anhydrous glycol and 625 kg of mixed acid containing HNO
3 41%, H
2SO
4 58% & water 1% were 222.2 kg of NGc at nitrating temp of 10-12° and only 218.3 kg at 29-30°. This means 90.6% of theory, as compared to 93.6% with NG.
- C2H4(OH)2 + 2 HNO3 → C2H4(ONO2)2 + 2 H2O
or through the reaction of ethylene oxide and dinitrogen pentoxide:
- C2H4O + N2O5 → C2H4(ONO2)2
2) Direct Production of NGc from Gaseous Ethylene.
3) Preparation of NGc from Ethylene Oxide.
4) Preparation of NGc by method of Messing from ethylene through chlorohydrin & ethylene oxide.
5) Preparation of NGc by duPont method.
Properties
editPhysical properties
editEthylene glycol dinitrate is a colorless volatile liquid when in pure state, but is yellowish when impure.
Molar weight 152.07, N 18.42%, OB to CO2 0%, OB to CO +21%; colorless volatile liquid when in pure state; yellowish liquid in crude state; sp gr 1.488 at 20/4° or 1.480 at 25°; n_D 1.4452 at 25° or 1.4472 at 20°; freezing point -22.75° (versus +13.1° for NG); frozen point given in[7] is -22.3°; boiling point 199° at 760mm Hg (with decomposition).
Brisance by lead block compression (Hess crusher test) is 30.0 mm, versus 18.5 mm for NG and 16 mm for TNT (misleading, needs to give exact density and mass of explosive (25 or 50 g). Brisance by sand test, determined in mixtures with 40% kieselguhr, gave for NGc mixtures slightly higher results then with those containing NG.
Chemical properties
editWhen ethylene glycol dinitrate is rapidly heated to 215 °C, it explodes; this is preceded by partial decomposition similar to that of nitroglycerin. EGDN has a slightly higher brisance than nitroglycerin.
Ethylene glycol dinitrate reacts violently with potassium hydroxide, yielding ethylene glycol and potassium nitrate:
- C2H2(ONO2)2 + 2 KOH → C2H2(OH)2 + 2 KNO3
Other
editEGDN was used in manufacturing explosives to lower the freezing point of nitroglycerin, in order to produce dynamite for use in colder weather. Due to its volatility it was used as a detection taggant in some plastic explosives, e.g. Semtex, to allow more reliable explosive detection, until 1995 when it was replaced by dimethyldinitrobutane. It is considerably more stable than glyceryl trinitrate owing to the lack of secondary hydroxyl groups in the precursor polyol.
Like other organic nitrates, ethylene glycol dinitrate is a vasodilator.
See also
editReferences
edit- ^ Jump up to: a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0273". National Institute for Occupational Safety and Health (NIOSH).
- ^ https://en.wiki.x.io/wiki/TNT_equivalent#Relative_effectiveness_factor }}
- ^ L. Henry (1870) "Untersuchungen über die Aetherderivate der mehratomigen Alkohole und Säuren (Vierter Theil.)" (Investigations of ether derivatives of polybasic alcohols and polyprotic acids (fourth part)), Berichte der deutschen chemischen Gesellschaft, 3 : 529–533.
- ^ Wieland, H.; Sakellarios, E. (1920). "Die Nitrierung des Äthylens" [The nitration of ethylene]. Berichte der Deutschen Chemischen Gesellschaft. 53 (2): 201–210. doi:10.1002/cber.19200530211.
- ^ Jump up to: a b Rinkenbach, W. H. (1926). "The Properties of Glycol Dinitrate". Industrial and Engineering Chemistry. 18 (11): 1195–1197. doi:10.1021/ie50203a027.
- ^ A. Kekulé (1869) "Ueber eine Verbindung des Aethylens mit Salpetersäure" (On a compound of ethylene with nitric acid), Berichte der deutschen chemischen Gesellschaft, 2 : 329–330.
- ^ Curme, G. O.; Johnston, F., eds. (1952). Glycols. American Chemical Society Monograph. Vol. 114. Reinhold. pp. 65–7, 130–134, 312. OCLC 558186858.