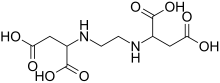
Ethylenediamine-N,N'-disuccinic acid (EDDS) is an aminopolycarboxylic acid. It is a colourless solid that is used as chelating agent that may offer a biodegradable alternative to EDTA, which is currently used on a large scale in numerous applications.
| |
| Names | |
|---|---|
| IUPAC name
Ethylenediamine-N,N′-disuccinic acid[citation needed]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | N,N'-ethylenediamine+disuccinic+acid |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H16N2O8 | |
| Molar mass | 292.244 g·mol−1 |
| Density | 1.44 g mL−1 |
| Melting point | 220 to 222 °C (428 to 432 °F; 493 to 495 K) |
| Acidity (pKa) | 2.4 |
| Basicity (pKb) | 11.6 |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−1.9541–−1.9463 MJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−4.2755–−4.2677 MJ mol−1 |
| Related compounds | |
Related alkanoic acids
|
EDTA |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure and properties
editEDDS has two chiral centers, and as such three stereoisomers.[1] These are the enantiomeric (R,R) and (S,S) isomers and the achiral meso (R,S) isomer. As a biodegradable replacement for EDTA, only the (S,S) stereoisomer is of interest. The (R,S) and (R,R) stereoisomers are less biodegradable, whereas the (S,S) stereoisomer has been shown to be very effectively biodegraded even in highly polluted soils.[2]
Synthesis
editEDDS was first synthesized from maleic acid and ethylenediamine.[3][4] Some microorganisms have been manipulated for industrial-scale synthesis of (S,S)-EDDS from ethylenediamine and fumaric acid or maleic acid, which proceeds as follows:[5]
From aspartic acid
edit(S,S)-EDDS is produced stereospecifically by the alkylation of an ethylenedibromide with L-aspartic acid. Racemic EDDS is produced by the reaction of ethylenediamine with fumaric acid or maleic acid.
Coordination chemistry
editIn comparing the effectiveness of (S,S)-EDDS versus EDTA as chelating agents for iron(III):
| Formation reaction | Formation constant |
| [Fe(H2O)6]3+ + (S,S)-EDDS4− → Fe[(S,S)-EDDS]− + 6 H2O | KEDDS = 1020.6 |
| [Fe(H2O)6]3+ + EDTA4− → Fe(EDTA)− + 6 H2O | KEDTA = 1025.1 |
Because of the lower stability for [Fe(S,S)-EDDS]−, the useful range being roughly 3<pH(S,S)-EDDS<9 and 2<pHEDTA<11. However, this range is sufficient for most applications.[6]
Another comparison that can be made between (S,S)-EDDS and EDTA is the structure of the chelated complex. EDTA’s six donor sites form five five-membered chelate rings around the metal ion, four NC2OFe rings and one C2N2Fe ring. The C2N2Fe ring and two of NC2OFe rings define a plane, and two NC2OFe rings are perpendicular to the plane that contains the C2-symmetry axis. The five-membered rings are slightly strained. EDDS’s six donor sites form both five- and six-membered chelate rings around the metal ion: two NC2OFe rings, two NC3OFe rings, and one C2N2Fe ring. Studies of the crystal structure of the Fe[(S,S)-EDDS]− complex show that the two five-membered NC3OFe rings project out of the plane of the complex, reducing the equatorial ring strain that exists in the Fe[EDTA]− complex.[7] The complex also has C2 symmetry.
Uses
edit(S,S)-EDDS is a biodegradable chelating agent that offers an alternative to EDTA, of which 80 million kilograms are produced annually. Under natural conditions, EDTA has been found to convert to ethylenediaminetriacetic acid and then cyclize to the diketopiperazine, which accumulates in the environment as a persistent organic pollutant.[8][9] (S,S)-EDDS was developed commercially as a biodegradable chelator and stabilizing agent in detergent and cosmetic formulations.[10] When EDDS is applied in chemical-enhanced soil remediation in excessive case (e.g., when applied for ex-situ soil washing), higher extraction efficiency for heavy metals can be achieved and the amount of extraction is less independent with the EDDS dosage;[11] On the other hand, during soil remediation which involves continuous flushing, metal extraction is often limited by the amount of EDDS. Under EDDS deficiency, initial unselective extraction of heavy metals was observed, followed by heavy metal exchange and re-adsorption of heavy metals that have lower stability constant with EDDS.[12]
External links
editReferences
edit- ^ Neal, J. A.; Rose, N. J. (1968). "Stereospecific Ligands and Their Complexes. I. A Cobalt(III) Complex of Ethylenediaminedisuccinic Acid". Inorganic Chemistry. 7 (11): 2405–2412. doi:10.1021/ic50069a043.
- ^ Tandy, S.; Ammann, A.; Schulin, R.; Nowack, B. (2006). "Biodegredation and speciation of residual SS-ethylenediaminedisuccinic acid (EDDS) in soil solution left after soil washing". Environmental Pollution. 142 (2): 191–199. doi:10.1016/j.envpol.2005.10.013. PMID 16338042.
- ^ Barbier, M.; et al. (1963). "Synthese und Eigenschaften eines Analogen des Lycomarasmins und der Aspergillomarasmine". Liebigs Annalen. 668: 132–138. doi:10.1002/jlac.19636680115.
- ^ US patent 3158635, Kezerian, Charles; Ramsey, William M., "Bisadducts of diamines and unsaturated acids", issued 1964-11-24
- ^ Takahashi, R.; et al. (1999). "Production of (S,S)-Ethylenediamine-N,N'-disuccinic Acid from Ethylenediamine and Fumaric Acid by Bacteria". Biosci. Biotechnol. Biochem. 63 (7): 1269–1273. doi:10.1271/bbb.63.1269. PMID 27380235.
- ^ Orama, M.; Hyvönen, H.; Saarinen, H.; Aksela, R. (2002). "Complexation of [S,S] and mixed stereoisomers of N,N'-ethylenediaminedisuccinic acid (EDDS) with Fe(III), Cu(II), Zn(II) and Mn(II) ions in aqueous solution". J. Chem. Soc., Dalton Trans. (24): 4644–4648. doi:10.1039/b207777a.
- ^ Pavelčík, F; Majer, J. (1978). "The crystal and molecular structure of lithium [(S,S)-N,N'-ethylenediaminedisuccinato]cobaltate(III) trihydrate". Acta Crystallographica B. 34 (12): 3582–3585. doi:10.1107/S0567740878011644.
- ^ Yuan, Z.; VanBriesen, J. M. (2006). "The Formation of Intermediates in EDTA and NTA Biodegradation". Environmental Engineering Science. 23 (3): 533–544. doi:10.1089/ees.2006.23.533.
- ^ Yip, T.C.M.; Tsang, D.C.W.; Ng, K.T.W.; Lo, I.M.C. (2009). "Kinetic interactions of EDDS with soils. 1. Metal resorption and competition under EDDS deficiency". Environ. Sci. Technol. 43 (3): 831–836. Bibcode:2009EnST...43..831Y. doi:10.1021/es802030k. PMID 19245023.
- ^ Schowanek, D.; Feijtel, T.C.J.; Perkins, C.M.; Hartman, F.A.; Federle, T.W.; Larson, R.J. (1997). "Biodegradation of (S,S), (R,R) and mixed stereoisomers of ethylene diamine disuccinic acid (EDDS), a transition metal chelator". Chemosphere. 34 (11): 2375–2391. doi:10.1016/S0045-6535(97)00082-9. PMID 9192467.
- ^ Yip, T.C.M.; Tsang, D.C.W.; Ng, K.T.W.; Lo, I.M.C. (2009). "Empirical modeling of heavy metal extraction by EDDS from single-metal and multi-metal contaminated soils". Chemosphere. 74 (2): 301–307. Bibcode:2009Chmsp..74..301Y. doi:10.1016/j.chemosphere.2008.09.006. PMID 18851868.
- ^ Tsang, D.C.W.; Yip, T.C.M.; Lo, I.M.C. (2009). "Kinetic interactions of EDDS with soils. 2. Metal-EDDS complexes in uncontaminated and metal-contaminated soils". Environ. Sci. Technol. 43 (3): 837–842. Bibcode:2009EnST...43..837T. doi:10.1021/es8020292. PMID 19245024.