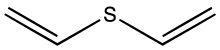
Divinyl sulfide is the organosulfur compound with the formula S(CH=CH2)2. A colorless liquid with a faint odor, it is found in some species of Allium.[1]
| |
| Names | |
|---|---|
| Preferred IUPAC name
(Ethenylsulfanyl)ethene | |
| Other names
vinyl sulfide, DVS
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6S | |
| Molar mass | 86.15 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.9098 g/cm3 (20 °C) |
| Melting point | 20 °C (68 °F; 293 K) |
| Boiling point | 84 °C (183 °F; 357 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editDivinyl sulfide is formed from hydrogen sulfide and acetylene.[2] Divinylsulfide can arise when inadvertently when acetylene is generated by hydrolysis of technical-grade calcium carbide contaminated with calcium sulfide.[3]
Divinylsulfide was first prepared in 1920 by the reaction of bis(2-chloroethyl)sulfide with sodium ethoxide:[3]
- (ClCH2CH2)2S + 2 NaOEt → (CH2=CH)2S + 2 EtOH + 2 NaCl
Monovinyl sulfides
editWith the formula CH2=CHSR, a variety of monovinyl sulfides are known. They can arise by the dehydrohalogenation of -2-haloethyl phenyl sulfides.[4] One example is phenyl vinyl sulfide.[5][6] Alkyl ketones react with thiols in the presence of phosphorus pentoxide to give vinyl sulfides:[7]
- RSH + CH3C(O)R' → CH2=C(SR)R' + H2O
References
edit- ^ "Divinyl sulfide (FDB012121)". FooDB.
- ^ Trotuş, Ioan-Teodor; Zimmermann, Tobias; Schüth, Ferdi (2014). "Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited". Chemical Reviews. 114 (3): 1761–1782. doi:10.1021/cr400357r. PMID 24228942.
- ^ a b Boris A. Trofimov; S. V. Amosova (1984). "Divinyl Sulfide: Synthesis, Properties, and Applications". Sulfur Reports. 3 (9): 323–393. doi:10.1080/01961778408082463.
- ^ Nina A. Nedolya; Boris A. Trofimov (1994). "Sulfur-Containing Vinyl Ethers". Sulfur Reports. 15 (2): 237–316. doi:10.1080/01961779408048961.
- ^ Leo A. Paquette; Richard V. C. Carr (1986). "Phenyl Vinyl Sulfone and Sulfoxide". Organic Syntheses. 64: 157. doi:10.15227/orgsyn.064.0157.
- ^ Daniel S. Reno; Richard J. Pariza (1997). "Phenyl Vinyl Sulfide". Organic Syntheses. 74: 124. doi:10.15227/orgsyn.074.0124.
- ^ Trost, Barry M.; Lavoie, Alvin C. (1983). "Enol thioethers as enol substitutes. An alkylation sequence". Journal of the American Chemical Society. 105 (15): 5075–5090. doi:10.1021/ja00353a037.