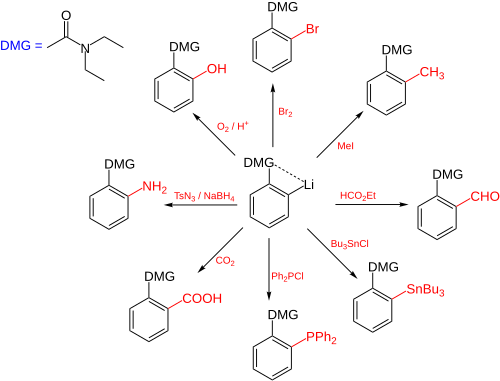
Directed ortho metalation (DoM) is an adaptation of electrophilic aromatic substitution in which electrophiles attach themselves exclusively to the ortho- position of a direct metalation group or DMG through the intermediary of an aryllithium compound.[1] The DMG interacts with lithium through a hetero atom. Examples of DMG's are the methoxy group, a tertiary amine group and an amide group.The compound can be produced by directed lithiation of anisole.[2]

The general principle is outlined in scheme 1. An aromatic ring system with a DMG group 1 interacts with an alkyllithium such as n-butyllithium in its specific aggregation state (hence (R-Li)n) to intermediate 2 since the hetero atom on the DMG is a Lewis base and lithium the Lewis acid. The very basic alkyllithium then deprotonates the ring in the nearest ortho- position forming the aryllithium 3 all the while maintaining the acid-base interaction. An electrophile reacts in the next phase in an electrophilic aromatic substitution with a strong preference for the lithium ipso position replacing the lithium atom.
Ordinary electrophilic substitutions with an activating group show preference for both the ortho and para position, this reaction demonstrates increased regioselectivity because the ortho position alone is targeted.
This reaction type was reported independently by Henry Gilman and Georg Wittig around 1940.[3][4]
Examples
editDOM has traditionally been applied to tertiary anilines and benzyl amines.[5][6]
The method has also been applied to the synthesis of enantiopure benzyl amines[7] in scheme 3,[8] which involves ortho-lithiation of tert-butyl phenyl sulfoxide. On approach to the lithium intermediate, the bulky tosyl group on the imine electrophile is responsible for the asymmetric induction taking place.
In another application[9] DOM is applied in placing a bulky tert-butyl group in an ortho position (scheme 4). The lithiation is a nucleophilic aromatic substitution and the subsequent reaction to the sulfoxide an electrophilic aromatic substitution. In the final step tert-butyllithium acts as a nucleophile in another nucleophilic aromatic substitution through an anionic intermediate.
DoM has also been applied combined with a Suzuki reaction in a one-pot synthesis:[10][11]
Thiophenol derivatives
editDOM has also been used with thiophenols to prepare compounds that are useful as hindered ligands.[12]
Related reaction
editDirected metallation is not limited to lithium intermediates or even to an ortho preference. In one study [13] it is found that the reaction product of N,N-dimethylaniline with a complex of TMEDA, sodium salt of TMP and di-tert-butylzinc is a meta zincated complex as a stable crystalline compound. This complex reacts with electrophilic iodine to N,N-dimethyl-3-iodoaniline:[14]
References
edit- ^ Snieckus, Victor (September 1990). "Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics". Chemical Reviews. 90 (6): 879–933. doi:10.1021/cr00104a001.
- ^ Gschwend, Heinz W.; Rodriguez, Herman R. (1979). "Heteroatom-Facilitated Lithiations". Organic Reactions. pp. 1–360. doi:10.1002/0471264180.or026.01. ISBN 0471264180.
- ^ Relative Reactivities of Organometallic Compounds. XX.* Metalation Henry Gilman, Robert L. Bebb J. Am. Chem. Soc.; 1939; 61(1); 109-112. doi:10.1021/ja01870a037
- ^ G. Wittig et al. Chem. Ber. 1940, 73, 1197
- ^ El-Hiti, Gamal A.; Smith, Keith; Hegazy, Amany S.; Alshammari, Mohammed B.; Masmali, Ali (2015). "Directed lithiation of simple aromatics and heterocycles for synthesis of substituted derivatives". Arkivoc. 2015: 19–48. doi:10.3998/ark.5550190.p008.744 (inactive 1 November 2024).
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ J. V. Hay And T. M. Harris "Dimethylamino-5-methylphenyl)diphenylcarbinol" Org. Synth. 1973, volume 53, 56. doi:10.15227/orgsyn.053.0056
- ^ ortho-Metalation of Enantiopure Aromatic Sulfoxides and Stereocontrolled Addition to Imines Nicolas Le Fur, Ljubica Mojovic, Nelly Plé, Alain Turck, Vincent Reboul, and Patrick Metzner J. Org. Chem.; 2006; 71(7) pp 2609 - 2616; Abstract
- ^ Scheme 3. Reaction scheme: reaction of iodobenzene with n-butyllithium and (S)-tert-butyl tert-butanethiosulfinate to enantiopure an sulfoxide followed by DOM reaction initiated again by n-butyllithium with electrophilic N-tosylimine. The sulfoxide group is removed by hydrogenation with Raney nickel. ts is a tosyl group, ee stands for enantiomeric excess
- ^ Contra-Friedel–Crafts tert-butylation of substituted aromatic rings via directed metallation and sulfinylation Jonathan Clayden, Christopher C. Stimson and Martine Keenan Chemical Communications, 2006, 1393 - 1394 Abstract
- ^ Directed ortho Metalation-Boronation and Suzuki-Miyaura Cross Coupling of Pyridine Derivatives: A One-Pot Protocol to Substituted Azabiaryls Manlio Alessi, Andrew L. Larkin, Kevin A. Ogilvie, Laine A. Green, Sunny Lai, Simon Lopez, and Victor Snieckus J. Org. Chem.; 2007; 72(5) pp 1588 - 1594. doi:10.1021/jo0620359
- ^ In this sequence the starting material nicotinamide is lithiated, then reacted with triisopropoxyborane to a boronate ester, then reacted with pinacol and finally reacted with iodobenzene and Tetrakis(triphenylphosphine)palladium(0)
- ^ Directed ortho-lithiation of lithium thiophenolate. New methodology for the preparation of ortho-substituted thiophenols and related compounds Garret D. Figuly, Cynthia K. Loop, J. C. Martin J. Am. Chem. Soc.; 1989; 111 pp 654-658 doi:10.1021/ja00184a038. Ortho-Lithiothiophenol Equivalents: Generation, Reactions and Applications in Synthesis of Hindered Thiolate Ligands Eric Block, Venkatachalam Eswarakrishnan, Michael Gernon, Gabriel Ofori-Okai, Chantu Saha, Kaluo Tang, Jon Zubieta J. Am. Chem. Soc.; 1989; 111 pp 658-665. doi:10.1021/ja00184a039. Directed lithiation of arenethiols Keith Smith, Charles M. Lindsay, Gareth J. Pritchard J. Am. Chem. Soc.; 1989; 111 pp 665-669; doi:10.1021/ja00184a040. 2-Phosphino- and 2-Phosphinyl-benzenethiols: New Ligand Types Eric Block, Gabriel Ofori-Okai and Jon Zubieta J. Am. Chem. Soc.; 1989; 111 pp 2327-2329; doi:10.1021/ja00188a071. Co-complexes of ortho-dilithiated thiophenol or 2-trimethylsilylthiophenol with lithiated TMEDA molecules: synthesis, crystal structures and theoretical studies (TMEDA = N,N,N′,N′-tetramethylethylenediamine) Alexandra Hildebrand, Peter Lönnecke, Luminita Silaghi-Dumitrescu, Ioan Silaghi-Dumitrescu and Evamarie Hey-Hawkins Dalton Transactions; 2006; 967-974; doi:10.1039/B511827A
- ^ Directed meta-Metalation Using Alkali-Metal-Mediated Zincation David R. Armstrong, William Clegg, Sophie H. Dale, Eva Hevia, Lorna M. Hogg, Gordon W. Honeyman, Robert E. Mulvey Angewandte Chemie International Edition Volume 45, Issue 23, Pages 3775 - 3778 2006 doi:10.1002/anie.200600720
- ^ Solvent hexane reaction at room temperature. Selected bond lengths in 2: Zn-C bond 203.5 pm in plane with aryl plane, Na-C bond 269 pm at 76° to aryl plane