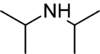
Diisopropylamine is a secondary amine with the chemical formula (Me2CH)2NH (Me = methyl). Diisopropylamine is a colorless liquid with an ammonia-like odor. Its lithium derivative, lithium diisopropylamide, known as LDA is a widely used reagent.
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-(Propan-2-yl)propan-2-amine | |
| Other names
Di(propan-2-yl)amine
N-Isopropylpropan-2-amine (Diisopropyl)amine (The name diisopropylamine is deprecated.) | |
| Identifiers | |
3D model (JSmol)
|
|
| 605284 | |
| ChemSpider | |
| ECHA InfoCard | 100.003.235 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1158 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H15N | |
| Molar mass | 101.193 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Fishy, ammoniacal |
| Density | 0.722 g mL−1 |
| Melting point | −61.00 °C; −77.80 °F; 212.15 K |
| Boiling point | 83 to 85 °C; 181 to 185 °F; 356 to 358 K |
| miscible[1] | |
| Vapor pressure | 9.3 kPa (at 20°C)[2] |
| Acidity (pKa) | 11.07 (in water) (conjugate acid) |
| Basicity (pKb) | 3.43[2] |
Refractive index (nD)
|
1.392–1.393 |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−173.6 to −168.4 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−4.3363 to −4.3313 MJ mol−1 |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H225, H302, H314, H332 | |
| P210, P280, P305+P351+P338, P310 | |
| NFPA 704 (fire diamond) | |
| Flash point | −17 °C (1 °F; 256 K) |
| 315 °C (599 °F; 588 K) | |
| Explosive limits | 1.1–7.1%[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
LC50 (median concentration)
|
1140 ppm (rat, 2 hr) 1000 ppm (mouse, 2 hr)[3] |
LCLo (lowest published)
|
2207 ppm (rabbit, 2.5 hr) 2207 ppm (guinea pig, 80 min) 2207 ppm (cat, 72 min)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 5 ppm (20 mg/m3) [skin][1] |
REL (Recommended)
|
TWA 5 ppm (20 mg/m3) [skin][1] |
IDLH (Immediate danger)
|
200 ppm[1] |
| Related compounds | |
Related amines
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Reactions and use
editDiisopropylamine is a common amine nucleophile in organic synthesis.[4] Because it is bulky, it is a more selective nucleophile than other similar amines, such as dimethylamine.[5]
It reacts with organolithium reagents to give lithium diisopropylamide (LDA). LDA is a strong, non-nucleophilic base[6]
The main commercial applications of diisopropylamine is as a precursor to the herbicide, diallate and triallate as well as certain sulfenamides used in the vulcanization of rubber.[7]
It is also used to prepare N,N-diisopropylethylamine (Hünig's base) by alkylation with diethyl sulfate.[8]
The bromide salt of diisopropylamine, diisopropylammonium bromide, is a room-temperature organic ferroelectric material.[9]
Preparation
editDiisopropylamine, which is commercially available, may be prepared by the reductive amination of acetone with ammonia using a modified copper oxide, generally copper chromite, as a catalyst:[10][11]
- NH3 + 2 (CH3)2CO + 2 H2 → C6H15N + 2 H2O
Diisopropylamine can be dried by distillation from potassium hydroxide (KOH) or drying over sodium wire.[12]: 186
Toxicity
editDiisopropylamine causes burns by all exposure routes. Inhalation of high concentrations of its vapor may cause symptoms like headache, dizziness, tiredness, nausea and vomiting.
References
edit- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0217". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b CID 7912 from PubChem
- ^ a b "Diisopropylamine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ John E. McMurry, Jack Melton (1977). "Conversion of Nitro to Carbonyl by Ozonolysis of Nitronates: 2,5-Heptanedione". Organic Syntheses. 56: 36. doi:10.15227/orgsyn.056.0036.
- ^ Denmark, Scott; Ryabchuk, Pavel; Min Chi, Hyung; Matviitsuk, Anastassia (2019). "Preparation of a Diisopropylselenophosphoramide Catalyst and its Use in Enantioselective Sulfenoetherification". Organic Syntheses. 96: 400–417. doi:10.15227/orgsyn.096.0400. PMC 8439352. PMID 34526731.
- ^ George M. Rubottom, John M. Gruber, Henrik D. Juve, Jr, , Dan A. Charleson (1986). "α-Hydroxy Ketones from the Oxidation of Enol Silyl Ethers with m-Chloroperbenzoic Acid: 6-Hydroxy- 3,5,5-trimethyl-2-cyclohexen-1-one". Organic Syntheses. 64: 118. doi:10.15227/orgsyn.064.0118.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (15 June 2000). Amines, Aliphatic. Wiley-VCH. doi:10.1002/14356007.a02_001. ISBN 978-3527303854. OL 9052422M.
{{cite encyclopedia}}:|journal=ignored (help) - ^ Hünig, Siegfried; Kiessel, Max (1 April 1958). "Spezifische Protonenacceptoren als Hilfsbasen bei Alkylierungs- und Dehydrohalogenierungsreaktionen" [Specific proton acceptors as auxiliary bases in alkylation and dehydrohalogenation reactions]. Chemische Berichte (in German). 91 (2). Wiley-VCH: 380–392. doi:10.1002/cber.19580910223. ISSN 0009-2940. OCLC 889715844.
- ^ Fu, Da-Wei; Cai, Hong-Ling; Liu, Yuanming; Ye, Qiong; Zhang, Wen; et al. (25 January 2013). "Diisopropylammonium Bromide Is a High-Temperature Molecular Ferroelectric Crystal". Science. 339 (6118): 425–428. Bibcode:2013Sci...339..425F. doi:10.1126/science.1229675. eISSN 1095-9203. ISSN 0036-8075. LCCN 17024346. OCLC 1644869. PMID 23349285. S2CID 12389978.
- ^ Löffler, Karl (1 April 1910). "Über eine neue Bildungsweise primärer und sekundärer Amine aus Ketonen" [About a new way of forming primary and secondary amines from ketones]. Berichte der Deutschen Chemischen Gesellschaft (in German). 43 (2): 2031–2035. doi:10.1002/cber.191004302145. ISSN 0365-9496. OCLC 219854722.
- ^ US 2686811, Willard Bull, "One-step process for preparing diisopropylamine"
- ^ Armarego, W. L. F.; Perrin, D. D. (16 October 1996). Purification of Laboratory Chemicals (4th ed.). Butterworth-Heinemann. ISBN 978-0750628396. LCCN 97109714. OCLC 762966259. OL 722457M.
