Caesium fluoride (cesium fluoride in American English) is an inorganic compound with the formula CsF. A hygroscopic white salt, caesium fluoride is used in the synthesis of organic compounds as a source of the fluoride anion.[5] The compound is noteworthy from the pedagogical perspective as caesium also has the highest electropositivity of all commonly available elements and fluorine has the highest electronegativity.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Caesium fluoride
| |
| Other names
Cesium fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.156 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CsF | |
| Molar mass | 151.903 g/mol[1] |
| Appearance | white crystalline solid |
| Density | 4.64 g/cm3[1] |
| Melting point | 703 °C (1,297 °F; 976 K)[1] |
| Boiling point | 1,251 °C (2,284 °F; 1,524 K) (2,284 °F; 1,524 K) |
| 573.0 g/100 mL (25 °C)[1] | |
| Solubility | Insoluble in acetone, diethyl ether, pyridine and ethanol 191 g/100 mL in methanol. |
| Basicity (pKb) | −744 kJ/mol |
| -44.5·10−6 cm3/mol[2] | |
Refractive index (nD)
|
1.477 |
| Structure | |
| cubic, cF8 | |
| Fm3m, No. 225[3] | |
a = 0.6008 nm[3]
| |
Lattice volume (V)
|
0.2169 nm3[3] |
Formula units (Z)
|
4 |
| Octahedral | |
| 7.9 D | |
| Thermochemistry | |
Heat capacity (C)
|
51.1 J/mol·K[4] |
Std molar
entropy (S⦵298) |
92.8 J/mol·K[4] |
Std enthalpy of
formation (ΔfH⦵298) |
-553.5 kJ/mol[4] |
Gibbs free energy (ΔfG⦵)
|
-525.5 kJ/mol[4] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H311, H315, H318, H331, H361f | |
| P201, P202, P260, P261, P264, P270, P271, P280, P281, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P311, P312, P321, P322, P330, P332+P313, P361, P362, P363, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions
|
Caesium chloride Caesium bromide Caesium iodide Caesium astatide |
Other cations
|
Lithium fluoride Sodium fluoride Potassium fluoride Rubidium fluoride Francium fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis and properties
editCaesium fluoride can be prepared by the reaction of caesium hydroxide (CsOH) with hydrofluoric acid (HF) and the resulting salt can then be purified by recrystallization. The reaction is shown below:
- CsOH + HF → CsF + H2O
Using the same reaction, another way to create caesium fluoride is to treat caesium carbonate (Cs2CO3) with hydrofluoric acid and again, the resulting salt can then be purified by recrystallization. The reaction is shown below:
- Cs2CO3 + 2 HF → 2 CsF + H2O + CO2
CsF is more soluble than sodium fluoride or potassium fluoride in organic solvents. It is available in its anhydrous form, and if water has been absorbed, it is easy to dry by heating at 100 °C for two hours in vacuo.[7] CsF reaches a vapor pressure of 1 kilopascal at 825 °C, 10 kPa at 999 °C, and 100 kPa at 1249 °C.[8]
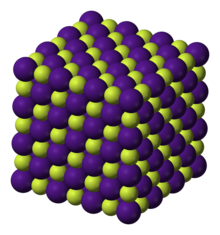
Structure
editCaesium fluoride has the halite structure, which means that the Cs+ and F− pack in a cubic closest packed array as do Na+ and Cl− in sodium chloride.[3] Unlike sodium chloride, caesium fluoride's anion is smaller than its cation, so it is the anion size that sterically inhibits larger coordination numbers than six under normally encountered conditions. A larger halide ion would allow for the eight-coordination seen in other caesium halide crystals.
Applications in organic synthesis
editBeing highly dissociated, CsF is a more reactive source of fluoride than related alkali metal salts. CsF is an alternative to tetra-n-butylammonium fluoride (TBAF) and TAS-fluoride (TASF).
As a base
editAs with other soluble fluorides, CsF is moderately basic, because HF is a weak acid. The low nucleophilicity of fluoride means it can be a useful base in organic chemistry.[9] CsF gives higher yields in Knoevenagel condensation reactions than KF or NaF.[10]
Formation of Cs-F bonds
editCaesium fluoride serves as a source of fluoride in organofluorine chemistry. Similarly to potassium fluoride, CsF reacts with hexafluoroacetone to form a stable perfluoroalkoxide salt.[11] It will convert electron-deficient aryl chlorides to aryl fluorides (Halex process), although potassium fluoride is more commonly used.
Deprotection agent
editDue to the strength of the Si–F bond, fluoride is useful for desilylation reactions, i.e., cleavage of Si-O bonds in organic synthesis.[12] CsF is commonly used for such reactions. Solutions of caesium fluoride in THF or DMF attack a wide variety of organosilicon compounds to produce an organosilicon fluoride and a carbanion, which can then react with electrophiles, for example:[10]
Precautions
editLike other soluble fluorides, CsF is moderately toxic.[13] Contact with acid should be avoided, as this forms highly toxic/corrosive hydrofluoric acid. The caesium ion (Cs+) and caesium chloride are generally not considered toxic.[14]
References
edit- ^ a b c d Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, Florida: CRC Press. p. 4.57. ISBN 1-4398-5511-0.
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, Florida: CRC Press. p. 4.132. ISBN 1-4398-5511-0.
- ^ a b c d Davey, Wheeler P. (1923). "Precision Measurements of Crystals of the Alkali Halides". Physical Review. 21 (2): 143–161. Bibcode:1923PhRv...21..143D. doi:10.1103/PhysRev.21.143.
- ^ a b c d Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, Florida: CRC Press. p. 5.10. ISBN 1-4398-5511-0.
- ^ Friestad, Gregory K.; Branchaud, Bruce P.; Navarrini, Walter; Sansotera, Maurizio (2007). "Cesium Fluoride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc050.pub2. ISBN 978-0-471-93623-7.
- ^ Senga, Ryosuke; Suenaga, Kazu (2015). "Single-atom electron energy loss spectroscopy of light elements". Nature Communications. 6: 7943. Bibcode:2015NatCo...6.7943S. doi:10.1038/ncomms8943. PMC 4532884. PMID 26228378. (Supplementary information)
- ^ Friestad, G. K.; Branchaud, B. P. (1999). Reich, H. J.; Rigby, J. H. (eds.). Handbook of Reagents for Organic Synthesis: Acidic and Basic Reagents. New York: Wiley. pp. 99–103. ISBN 978-0-471-97925-8.
- ^ Lide, D. R., ed. (2005). "Vapor Pressure" (PDF). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. p. 6.63. ISBN 0-8493-0486-5.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 82–83. ISBN 978-0-08-022057-4.
- ^ a b Fiorenza, M; Mordini, A; Papaleo, S; Pastorelli, S; Ricci, A (1985). "Fluoride ion induced reactions of organosilanes: the preparation of mono and dicarbonyl compounds from β-ketosilanes". Tetrahedron Letters. 26 (6): 787–788. doi:10.1016/S0040-4039(00)89137-6.
- ^ Evans, F. W.; Litt, M. H.; Weidler-Kubanek, A. M.; Avonda, F. P. (1968). "Formation of adducts between fluorinated ketones and metal fluorides". Journal of Organic Chemistry. 33 (5): 1837–1839. doi:10.1021/jo01269a028.
- ^ Smith, Adam P.; Lamba, Jaydeep J. S. & Fraser, Cassandra L. (2002). "Efficient Synthesis of Halomethyl-2,2'-bipyridines: 4,4'-Bis(chloromethyl)-2,2'-bipyridine". Organic Syntheses. 78: 82; Collected Volumes, vol. 10, p. 107.
- ^ "MSDS Listing for cesium fluoride". hazard.com. April 27, 1993. Archived from the original on 2012-02-09. Retrieved September 7, 2007.
- ^ "MSDS Listing for cesium chloride". hazard.com. JT Baker. January 16, 2006. Archived from the original on 2012-03-13. Retrieved September 7, 2007.
