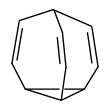
Bullvalene is a hydrocarbon with the chemical formula C10H10. The molecule has a cage-like structure formed by the fusion of one cyclopropane and three cyclohepta-1,4-diene rings. Bullvalene is unusual as an organic molecule due to the C−C and C=C bonds forming and breaking rapidly on the NMR timescale; this property makes it a fluxional molecule.[1]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Tricyclo[3.3.2.02,8]deca-3,6,9-triene | |||
| Other names
Bullvalen
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H10 | |||
| Molar mass | 130.19 g/mol | ||
| Melting point | 96 °C (205 °F; 369 K) | ||
| Boiling point | decomposition at about 400 °C (752 °F; 673 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Stereodynamics
editThe bullvalene molecule is a cyclopropane platform with three vinylene arms conjoined at a methine group. This arrangement enables a degenerate Cope rearrangement with the result that all carbon atoms and hydrogen atoms appear equivalent on the NMR timescale. At room temperature the 1H NMR signals average to a rounded peak at 5.76 ppm.[2] At lower temperatures the peak broadens into a mound-like appearance, and at very low temperatures the fluxional behavior of bullvalene is reduced, allowing for 4 total signals to be seen. This pattern is consistent with an exchange process whose rate k is close to the frequency separation of the four contributing resonances. The number of possible valence tautomers of a bullvalene with ten distinguishable positions is 10!/3 = 1,209,600.
Synthesis
editIn 1963, G. Schröder produced bullvalene by photolysis of a dimer of cyclooctatetraene. The reaction proceeds with expulsion of benzene.[3]
In 1966 W. von Eggers Doering and Joel W. Rosenthal synthesized it by the photochemical rearrangement of cis-9,10-dihydronaphthalene.[4]
Related compounds
editBullvalones
editIn bullvalones one vinyl group in one of the arms in bullvalene is replaced by a keto group on a methylene bridge. In this way it is possible to activate the fluxional state by adding base and deactivate it again by removing the base:[5]
Compound 1 in scheme 2 is not a fluxional molecule but by adding base (sodium methoxide in methanol) the ketone converts to the enolate 2 and the fluxional state is switched on. Deuterium labeling is possible forming first 3 a then a complex mixture with up to 7 deuterium atoms, compound 4 being just one of them.
Semibullvalene
editIn semibullvalene (C8H8), one ethylene arm is replaced by a single bond. The compound was first prepared by photolysis of barrelene in isopentane with acetone as a photosensitizer in 1966.[6]
Semibullvalene exists only as two valence tautomers (2a and 2b in scheme 3) but in this molecule the Cope rearrangement takes place even at -110 °C, a temperature at which this type of reaction is ordinarily not possible.
One insight into the reaction mechanism for this photoreaction is given by an isotope scrambling experiment.[7] The 6 vinylic protons in barrelene 1 are more acidic than the two bridgehead protons and therefore they can be replaced by deuterium with N-deuteriocyclohexylamide. Photolysis of 2 results in the initial formation of a biradical intermediate with a cyclopropane ring formed. This product rearranges to a second intermediate with a more favorable allylic radical as two mesomers. Intersystem crossing and radical recombination results in equal quantities of semibullvalenes 3 and 4. The new proton distribution with allylic, vinylic and cyclopropanyl protons determined with proton NMR confirms this model. As noted, the conversion of barrelene to semibullvalene is a di-π-methane rearrangement.
A synthetic procedure for alkylated semibullvalenes published in 2006 is based on cyclodimerisation of a substituted 1,4-dilithio-1,3-butadiene with copper(I) bromide.[8] At 140 °C the ethylated semibullvalene isomerises to the cyclooctatetraene derivative.
Barbaralane
editIn barbaralane, one ethylene arm is replaced by a methylene bridge and the dynamics are comparable to that of semibullvalene. There is also an intermediate ketone in bullvalene synthesis called "barbaralone". Both are named after Barbara M. Ferrier,[9] (1932–2006) professor of the Department of Biochemistry and Biomedical Sciences at McMaster University.[10]
Origin of the name
editThe name bullvalene is derived from the nickname of one of the scientists who predicted its properties in 1963 and the underlying concept of valence tautomerism,[11] William "Bull" Doering.[12][13] According to Klärner in 2011, the weekly seminars organised by Doering were secretly called "Bull sessions" by PhD students and postdocs and "were feared by those who were poorly prepared".[14] The name was bestowed on the molecule, in 1961, by two of Doering's Yale graduate students, Maitland Jones Jr and Ron Magid. The name celebrates Bill Doering's well-known nickname and was chosen to rhyme with fulvalene, a molecule of great interest to the research group.[15]
References
edit- ^ Addison Ault (2001). "The Bullvalene Story. The Conception of Bullvalene, a Molecule That Has No Permanent Structure". Journal of Chemical Education. 78 (7): 924. Bibcode:2001JChEd..78..924A. doi:10.1021/ed078p924.
- ^ Oth, J.; Mullen, K.; Gilles, J.; Schröder, G. (1974). "Comparison of 13C- and 1H- magnetic resonance spectroscopy as techniques for the quantitative investigation of dynamic processes. The Cope rearrangement in bullvalene". Helv Chim Acta. 57 (5): 1415–1433. doi:10.1002/hlca.19740570518.
- ^ Schröder, Gerhard (1963). "Preparation and Properties of Tricyclo[3,3,2,04,6]deca-2,7,9-triene (Bullvalene)". Angewandte Chemie International Edition in English. 2 (8): 481–482. doi:10.1002/anie.196304814. ISSN 0570-0833.
- ^ Von Eggers Doering, W.; Rosenthal, Joel W. (1966). "9,10-Dihydronaphthalene. Formation from Bullvalene and Nenitzescu's Hydrocarbon, Thermal Reorganization, and Photorearrangement to Bullvalene". J. Am. Chem. Soc. 88 (9): 2078–2079. doi:10.1021/ja00961a061.
- ^ Lippert, A. R.; Kaeobamrung, J.; Bode, J. W. (2006). "Synthesis of Oligosubstituted Bullvalones: Shapeshifting Molecules Under Basic Conditions". J. Am. Chem. Soc. 128 (46): 14738–14739. doi:10.1021/ja063900+. PMID 17105247.
- ^ Zimmerman, H. E.; Grunewald, G. L. (1966). "The Chemistry of Barrelene. III. A Unique Photoisomerization to Semibullvalene". J. Am. Chem. Soc. 88 (1): 183–184. doi:10.1021/ja00953a045.
- ^ Zimmerman, H. E.; Binkley, R. W.; Givens, R. S.; Sherwin, M. A. (1967). "Mechanistic Organic Photochemistry. XXIV. The Mechanism of the Conversion of Barrelene to Semibullvalene. A General Photochemical Process". J. Am. Chem. Soc. 89 (15): 3932–3933. doi:10.1021/ja00991a064.
- ^ Wang, C.; Yuan, J.; Li, G.; Wang, Z.; Zhang, S.; Xi, Z. (2006). "Metal-Mediated Efficient Synthesis, Structural Characterization, and Skeletal Rearrangement of Octasubstituted Semibullvalenes". J. Am. Chem. Soc. 128 (14): 4564–4565. doi:10.1021/ja0579208. PMID 16594680.
- ^ Alex Nickon, Ernest F. Silversmith, Organic Chemistry: The Name Game: Modern Coined Terms and Their Origins, p. 133, Pergamon Press, 1987.
- ^ A tribute to professor emeritus Barbara Ferrier Archived 2017-01-02 at the Wayback Machine, McMaster University, 6 January 2006
- ^ Doering, W. von E.; Roth, W. R. (1963). "A Rapidly Reversible Degenerate Cope Rearrangement : Bicyclo[5.1.0]octa-2,5-diene". Tetrahedron. 19 (5): 715–737. doi:10.1016/S0040-4020(01)99207-5.[dead link]
- ^ Ault, Addison (2001). "The Bullvalene Story. The Conception of Bullvalene, a Molecule That Has No Permanent Structure". J. Chem. Educ. 78 (7): 924. Bibcode:2001JChEd..78..924A. doi:10.1021/ed078p924.
- ^ Author Ault (2001) also suggests the name stems from BS because of an unimpressed grad student
- ^ Klärner, F.-G. (2011), William von Eggers Doering (1917–2011). Angewandte Chemie International Edition, 50: 2885–2886. doi:10.1002/anie.201100453
- ^ Nickon, A.; Silversmith, E. F. Organic Chemistry: The Name Game; Pergamon: New York, 1972; p 131.