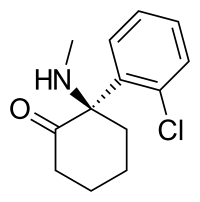
Arketamine (developmental code names PCN-101, HR-071603), also known as (R)-ketamine or (R)-(−)-ketamine, is the (R)-(−) enantiomer of ketamine.[1][2][3] Similarly to racemic ketamine and esketamine, the S(+) enantiomer of ketamine, arketamine is biologically active; however, it is less potent as an NMDA receptor antagonist and anesthetic and thus has never been approved or marketed for clinical use as an enantiopure drug.[1][3] Arketamine is currently in clinical development as a novel antidepressant.[4][5]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | PCN-101; HR-071603 |
| Addiction liability | Moderate |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H16ClNO |
| Molar mass | 237.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Relative to esketamine, arketamine possesses 4 to 5 times lower affinity for the PCP site of the NMDA receptor.[2][6] In accordance, arketamine is significantly less potent than racemic ketamine and especially esketamine in terms of anesthetic, analgesic, and sedative-hypnotic effects.[6] Racemic ketamine has weak affinity for the sigma receptor, where it acts as an agonist, whereas esketamine binds negligibly to this receptor, and so the sigma receptor activity of racemic ketamine lies in arketamine.[7] It was suggested that this action of arketamine may play a role in the hallucinogenic effects of racemic ketamine and that it may be responsible for the lowering of the seizure threshold seen with racemic ketamine.[7] However several subsequent studies have indicated that esketamine is more likely to induce dissociative events,[8][9] while studies in patients undergoing electroconvulsive therapy suggested that esketamine is a potent inducer of seizures.[10] Esketamine inhibits the dopamine transporter about 8-fold more potently than does arketamine, and so is about 8 times more potent as a dopamine reuptake inhibitor.[11] Arketamine and esketamine possess similar potency for interaction with the muscarinic acetylcholine receptors.[12]
Novel antidepressant
editArketamine appears to be more effective as a rapid-acting antidepressant than esketamine in preclinical research.[13]
In rodent studies, esketamine produced hyperlocomotion, prepulse inhibition deficits, and rewarding effects, while arketamine did not, in accordance with its lower potency as an NMDA receptor antagonist and dopamine reuptake inhibitor.[14] As such, arketamine may have a lower propensity for producing psychotomimetic effects and a lower abuse potential in addition to superior antidepressant efficacy.[14]
A study conducted in mice found that ketamine's antidepressant activity is not caused by ketamine inhibiting NMDAR, but rather by sustained activation of a different glutamate receptor, the AMPA receptor, by a metabolite, (2R,6R)-hydroxynorketamine; as of 2017 it was unknown if this was happening in humans.[15][16] Arketamine is an AMPA receptor agonist.[17]
Paradoxically, arketamine shows greater and longer-lasting rapid antidepressant effects in animal models of depression relative to esketamine.[13][18][14] It has been suggested that this may be due to the possibility of different activities of arketamine and esketamine and their respective metabolites at the α7-nicotinic receptor, as norketamine and hydroxynorketamine are potent antagonists of this receptor and markers of potential rapid antidepressant effects (specifically, increased mammalian target of rapamycin function) correlate closely with their affinity for it.[19][20][21] The picture is unclear however, and other mechanisms have also been implicated.[14]
Clinical development
editAs of November 2019, arketamine is under development for the treatment of depression under the developmental code names PCN-101 by Perception Neuroscience in the United States and HR-071603 by Jiangsu Hengrui Medicine in China.[22][4][5] Arketamine failed to show antidepressant effectiveness in a controlled phase 2a clinical trial.[23][24]
See also
editReferences
edit- ^ a b Ganellin CR, Triggle DJ (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1188–. ISBN 978-0-412-46630-4.
- ^ a b Yew DT (6 March 2015). Ketamine: Use and Abuse. Taylor & Francis. pp. 269–. ISBN 978-1-4665-8340-5.
- ^ a b Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, et al. (September 2016). "Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study". Biological Psychiatry. 80 (6): 424–431. doi:10.1016/j.biopsych.2015.10.018. PMID 26707087.
- ^ a b Hashimoto K (October 2019). "Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective". Psychiatry and Clinical Neurosciences. 73 (10): 613–627. doi:10.1111/pcn.12902. PMC 6851782. PMID 31215725.
- ^ a b "Arketamine - Jiangsu Hengrui Medicine". AdisInsight. Springer Nature Switzerland AG.
- ^ a b Barash P, Cullen BF, Stoelting RK, Cahalan M, Stock MC, Ortega R (28 March 2012). Clinical Anesthesia. Lippincott Williams & Wilkins. pp. 456–. ISBN 978-1-4511-4795-7.
- ^ a b Verster JC, Brady K, Galanter M, Conrod P, eds. (6 July 2012). Drug Abuse and Addiction in Medical Illness: Causes, Consequences and Treatment. Springer Science & Business Media. pp. 205–. ISBN 978-1-4614-3375-0.
- ^ Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J (February 1997). "Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET)". European Neuropsychopharmacology. 7 (1): 25–38. doi:10.1016/S0924-977X(96)00042-9. PMID 9088882. S2CID 26861697.
- ^ Engelhardt W (March 1997). "[Recovery and psychomimetic reactions following S-(+)-ketamine]". Der Anaesthesist. 46 (Suppl 1): S38–S42. doi:10.1007/pl00002463. PMID 9163277. S2CID 24966884.
- ^ Zavorotnyy M, Kluge I, Ahrens K, Wohltmann T, Köhnlein B, Dietsche P, et al. (December 2017). "S -ketamine compared to etomidate during electroconvulsive therapy in major depression". European Archives of Psychiatry and Clinical Neuroscience. 267 (8): 803–813. doi:10.1007/s00406-017-0800-3. PMID 28424861. S2CID 22725552.
- ^ Nishimura M, Sato K (October 1999). "Ketamine stereoselectively inhibits rat dopamine transporter". Neuroscience Letters. 274 (2): 131–134. doi:10.1016/s0304-3940(99)00688-6. PMID 10553955. S2CID 10307361.
- ^ Vuyk J, Schraag S (6 December 2012). Advances in Modelling and Clinical Application of Intravenous Anaesthesia. Springer Science & Business Media. pp. 270–. ISBN 978-1-4419-9192-8.
- ^ a b Zhang JC, Li SX, Hashimoto K (January 2014). "R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine". Pharmacology, Biochemistry, and Behavior. 116: 137–141. doi:10.1016/j.pbb.2013.11.033. PMID 24316345. S2CID 140205448.
- ^ a b c d Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. (September 2015). "R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects". Translational Psychiatry. 5 (9): e632. doi:10.1038/tp.2015.136. PMC 5068814. PMID 26327690.
- ^ Tyler MW, Yourish HB, Ionescu DF, Haggarty SJ (June 2017). "Classics in Chemical Neuroscience: Ketamine". ACS Chemical Neuroscience. 8 (6): 1122–1134. doi:10.1021/acschemneuro.7b00074. PMID 28418641.
- ^ Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. (May 2016). "NMDAR inhibition-independent antidepressant actions of ketamine metabolites". Nature. 533 (7604): 481–486. Bibcode:2016Natur.533..481Z. doi:10.1038/nature17998. PMC 4922311. PMID 27144355.
- ^ Yang C, Zhou W, Li X, Yang J (May 2012). "A bright future of researching AMPA receptor agonists for depression treatment". Expert Opinion on Investigational Drugs. 21 (5): 583–585. doi:10.1517/13543784.2012.667399. PMID 22375566. S2CID 19842307.
- ^ Hashimoto K (April 2014). "The R-Stereoisomer of Ketamine as an Alternative for Ketamine for Treatment-resistant Major Depression". Clinical Psychopharmacology and Neuroscience. 12 (1): 72–73. doi:10.9758/cpn.2014.12.1.72. PMC 4022771. PMID 24851126.
- ^ van Velzen M, Dahan A (July 2014). "Ketamine metabolomics in the treatment of major depression". Anesthesiology. 121 (1): 4–5. doi:10.1097/ALN.0000000000000286. PMID 24936919.
- ^ Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, et al. (July 2014). "(R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function". Anesthesiology. 121 (1): 149–159. doi:10.1097/ALN.0000000000000285. PMC 4061505. PMID 24936922.
- ^ Singh NS, Zarate CA, Moaddel R, Bernier M, Wainer IW (November 2014). "What is hydroxynorketamine and what can it bring to neurotherapeutics?". Expert Review of Neurotherapeutics. 14 (11): 1239–1242. doi:10.1586/14737175.2014.971760. PMC 5990010. PMID 25331415.
- ^ "Arketamine - ATAI Life Sciences". AdisInsight. 10 June 2024. Retrieved 23 October 2024.
- ^ Kim JW, Suzuki K, Kavalali ET, Monteggia LM (January 2024). "Ketamine: Mechanisms and Relevance to Treatment of Depression". Annu Rev Med. 75: 129–143. doi:10.1146/annurev-med-051322-120608. PMID 37729028.
- ^ "atai Life Sciences Announces Results from Phase 2a Trial of PCN-101 (R-ketamine) for Treatment-Resistant Depression". atai Life Sciences. 9 January 2023. Retrieved 23 October 2024.