Angustifodilactones are natural compounds isolated from Kadsura and showing some activity against HIV in the cell culture.[1]

| |
| Names | |
|---|---|
| IUPAC name
(1S,3aS,3bS,5S,5aS,10aS,11aR,13S,13aR)-5,13-Dihydroxy-1-{(1R)-1-hydroxy-1-[(2R)-5-(hydroxymethyl)-6-oxo-3,6-dihydro-2H-pyran-2-yl]ethyl}-3a,6,6,13a-tetramethyl-1,2,3,3a,3b,4,5,5a,6,12,13,13a-dodecahydro-8H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-8-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H42O8 | |
| Molar mass | 530.658 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
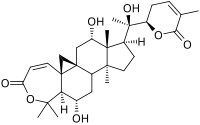
| |
| Names | |
|---|---|
| IUPAC name
(1S,3aS,3bS,5S,5aS,10aS,11aR,13S,13aR)-5,13-Dihydroxy-1-{(1R)-1-hydroxy-1-[(2R)-5-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl]ethyl}-3a,6,6,13a-tetramethyl-1,2,3,3a,3b,4,5,5a,6,12,13,13a-dodecahydro-8H-cyclopenta[5,6]cyclopropa[1,8a]naphtho[2,1-c]oxepin-8-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H42O7 | |
| Molar mass | 514.659 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
See also
editReferences
edit- ^ Sun, R; Song, HC; Wang, CR; Shen, KZ; Xu, YB; Gao, YX; Chen, YG; Dong, JY (2011). "Compounds from Kadsura angustifolia with anti-HIV activity". Bioorg Med Chem Lett. 21 (3): 961–5. doi:10.1016/j.bmcl.2010.12.055. PMID 21232955.