Anagyrine is a teratogenic alkaloid first isolated from (and named for) Anagyris foetida in the year 1885 by French biologists Hardy and Gallois.[6] A. foetida (family Fabaceae), the Stinking Bean Trefoil, is a highly toxic shrub native to the Mediterranean region, with a long history of use in folk medicine.[7][8] In the year 1939 Anagyrine was found by James Fitton Couch to be identical to an alkaloid present in many species belonging to the plant genus Lupinus (lupins).[9] The toxin can cause crooked calf disease if a cow ingests the plant during certain periods of pregnancy.
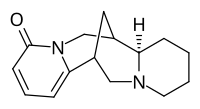 Skeletal structure of anagyrine
| |
 | |
| Names | |
|---|---|
| Systematic IUPAC name
(7Ξ,7aR,14Ξ)-7,7a,8,9,10,11,13,14-Octahydro-4H,6H-7,14-methanodipyrido[1,2-a:1′,2′-e][1,5]diazocin-4-one | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.215.995 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H20N2O | |
| Molar mass | 244.338 g·mol−1 |
| Density | 1.22 ±0.1 g/mL[5] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Background
editThe toxicity of certain species of Lupinus plants has been known for several years. The plant is very common in western North America and is sometimes used in feed for cattle if the toxicity of the given lupine is low enough. The toxicity of the plant comes from a variety of toxins, however out of these chemicals anagyrine is the most well known for causing crooked calf disease when ingested by cows.[10] The discovery of anagyrine was made in 1885 by French biologists Ernest Hardy (born Paris 1826) and N. Gallois, who isolated it from the highly toxic legume Anagyris foetida, while the earliest isolation of anagyrine from a lupinus plant was recorded in 1939.[11][9] The toxin can be found in growing leaf material in a young lupinus plant and in the flower and seed of a mature plant, though varying concentrations of the alkaloid are present throughout lupines that contain anagyrine.[10] The first correlation between anagyrine and crooked calf disease was made by Richard Keeler in 1973.[12] Recently there have been a few successful syntheses of anagyrine recorded, most notably one completed by Diane Gray and Timothy Gallagher.[13]
Toxicity
editAnagyrine causes crooked calf disease if 1.44 g/kg of the substance is ingested by the mother cow between days 40 and 70 of pregnancy. Out of the hundreds of varieties of lupinus plants, 23 (listed below) are known to contain high enough concentrations of anagyrine to be dangerous to cattle.[14][15] The IC50 of anagyrine is 132 μM at muscarinic receptors and 2,096 μM at nicotinic receptors.[16]
| Common Name | Scientific Name |
|---|---|
| Mountain Silvery Lupine | L. alpestris |
| Arctic Lupine | L. arcticus |
| Anderson's Lupine | L. andersonii |
| Spur Lupine | L. arbustus |
| Silvery Lupine | L. argenteus |
| Tail Cup Lupine | L. caudatus |
| unknown | L. cyaneus |
| Lunara Lupine | L. formosus |
| unknown | L. greenei |
| Broad-Leafed Lupine | L. latifolius |
| Spurred Lupine | L. laxiflorus |
| White-Leaved Lupine | L. leucophyllus |
| Nootka Lupine | L. nootaktensis |
| Plumes Lupine | L. onustus |
| Meadow Lupine | L. polyphyllus |
| Rusty Lupine | L. pusillus |
| Silky Lupine | L. sericeus |
| Yellow Lupine | L. sulphureus |
| Burke's Lupine | L. burkei |
| Seashore Lupine | L. littoralis |
| Pine Lupine | L. albicaulis |
| Tall Silvery Lupine | L. erectus |
| Mt. Rose Lupine | L. montigenus |
Symptoms
editKnown symptoms of crooked cow disease include arthrogryposis (permanently flexed joints), torticollis (twisting of the neck), scoliosis (curving of the spine), kyphosis (humpback), and cleft plate. It is thought that teratogenic alkaloids like anagyrine cause the deformities by sedating the fetus, causing it to remain fixed in an abnormal position as it grows.[10] Lasting malformations of the calf can occur even in mild poisonings of the cow because fetal movement depression persists much longer between doses of teratogenic alkaloids than the signs of toxicity in the cow.
Symptoms of the alkaloid being ingested by a cow include dyspnea, nervousness, grounding of teeth, depression, salivation, ataxia, spasms, head pressing tremors, seizures, coma, and sometimes death within days of ingestion. If the cattle do not die as a result of alkaloid ingestion, most make a complete recovery with no lasting signs of being poisoned.[17][16]
Mechanism of Action
editWhile the specific mechanism of action of anagyrine is unknown, the structure of anagyrine allows it to be mistaken for acetylcholine by certain receptors in living organisms. Anagyrine is thought to act as an acetylcholine agonist, increasing the amount of signal being sent to muscles in the organism's body, much like nicotine. Anagyrine interacts with nicotinic and muscarinic acetylcholine receptors, however it binds to muscarinic receptors 16 times more strongly, making it likely that the blocking of muscarinic acetylcholine receptors is what causes crooked calf disease.[16]
References
edit- ^ Turgunov, K.K.; Rakhimov, S.B.; Vinogradova, V.I.; Tashkhodjaev, B. (2015). "CSD Entry: UHUHOW". Cambridge Structural Database: Access Structures. Cambridge Crystallographic Data Centre. doi:10.5517/cc14ll5h. Retrieved 2022-07-28.
- ^ Turgunov, K. K.; Rakhimov, S. B.; Vinogradova, V. I.; Tashkhodjaev, B. (2015). "Crystal structure of anagyrine perchlorate". Acta Crystallogr. E. 71 (5): o343–o344. Bibcode:2015AcCrE..71O.343T. doi:10.1107/S2056989015007781. PMC 4420039. PMID 25995939. S2CID 22686042.
- ^ a b P.J. Linstrom and W.G. Mallard, Eds., NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg MD, 20899, doi:10.18434/T4D303, (retrieved April 21, 2017)
- ^ "3,4,5,6-Tetradehydrospartein-2-one." 3,4,5,6-Tetradehydrospartein-2-one | C15H20N2O | ChemSpider. Royal Society of Chemistry, n.d. Web. 25 Apr. 2017.
- ^ 486-89-5(ANAGYRINE) Product Description." ChemicalBook---Chemical Search Engine. N.p., n.d. Web. 21 Apr. 2017.
- ^ Hardy and Gallois, Comptes-rendus et Mémoires de la Société de Biologie, 13th June 1885
- ^ Pedanius Dioscorides, De materia medica Book 3: "Roots of Akanthoda (= Prickly Plants) No. 167 "Anaguris [Onaguris]" http://www.cancerlynx.com/BOOKTHREEROOTS.PDF
- ^ Partheil and Spassky, Apoth. Ztg. 1895, 10, 903 https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=JR9330000504&imageInfo.ImageIdentifier.Year=1933 Retrieved at 11.59 on 28/11/22.
- ^ a b Couch, James Fitton. "Lupine Studies. XIV.1 The Isolation of Anagyrine FromLupinus Laxiflorusvar.silvicola C. P. Smith." Journal of the American Chemical Society 61.12 (1939): 3327-328. https://pubs.acs.org/doi/pdf/10.1021/ja01267a027 Retrieved at 12.18 on 28/11/22.
- ^ a b c "AAHP Field Disease Investigation Unit (FDIU)." Crooked Calf. Washington State University: College of Veterinary Medicine, 2015. Web. 23 Apr. 2017.
- ^ Hardy, E., and N. Gallois. "Keeler, Richard F. "Lupin Alkaloids from Teratogenic and Nonteratogenic Lupins. I. Correlation of Crooked Calf Disease Incidence with Alkaloid Distribution Determined by Gas Chromatography." Teratology 7.1 (1973): 23-30. Wiley Online Library. Web." The Journal of the Chemical Society 54 (1888): n. pag. Abstract. The Journal of the Chemical Society (n.d.): n. pag. Print.
- ^ Keeler, Richard F. "Lupin Alkaloids from Teratogenic and Nonteratogenic Lupins. I. Correlation of Crooked Calf Disease Incidence with Alkaloid Distribution Determined by Gas Chromatography." Teratology 7.1 (1973): 23-30. Wiley Online Library. Web.
- ^ Gray, Diane, and Timothy Gallagher. "A Flexible Strategy for the Synthesis of Tri- and Tetracyclic Lupin Alkaloids: Synthesis of ( )-Cytisine, (±)-Anagyrine, and (±)-Thermopsine." Angewandte Chemie International Edition 45.15 (2006): 2419-423. Web.
- ^ Davis, A. M., and D. M. Stout. "Anagyrine in Western American Lupines." Journal of Range Management 1.39 (1986): n. pag. Web. 23 Apr. 2017.
- ^ Gupta, Ramesh C. Veterinary Toxicology: Basic and Clinical Principles. N.p.: Academic, 2007. Print.
- ^ a b c GUPTA, RAMESH C. Reproductive and Developmental Toxicology. Place of Publication Not Identified: ELSEVIER ACADEMIC, 2017. Print.
- ^ Schenck, Patricia. Saunders Comprehensive Review of the Navle: Pageburst Retail. Place of Publication Not Identified: Elsevier Saunders, 2009. Print.