An ameloblastic fibroma is a fibroma of the ameloblastic tissue, that is, an odontogenic tumor arising from the enamel organ or dental lamina. It may be either truly neoplastic or merely hamartomatous (an odontoma). In neoplastic cases, it may be labeled an ameloblastic fibrosarcoma in accord with the terminological distinction that reserves the word fibroma for benign tumors and assigns the word fibrosarcoma to malignant ones. It is more common in the first and second decades of life, when odontogenesis is ongoing, than in later decades. In 50% of cases an unerupted tooth is involved.
| Ameloblastic fibroma | |
|---|---|
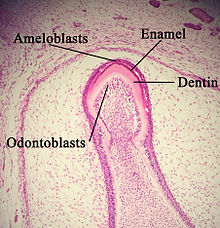 | |
| Histology of developing tooth with enamel, dentin, ameloblasts, and odontoblasts labeled. Tooth bud is in maturation/crown stage. | |
| Symptoms | Painless swelling of the jaw.[1] |
| Complications | Impacted tooth.[1] |
| Usual onset | Childhood or adolescence.[1] |
| Diagnostic method | Dental radiograph.[1] |
| Differential diagnosis | Ameloblastic fibro-odontoma or odontoma.[1] |
| Treatment | Surgical excision or curettage and removal of the effected teeth.[1] |
| Frequency | 2% of odontogenic tumors.[1] |
Histopathology alone is usually not enough to differentiate neoplastic cases from hamartomatous ones, because the histology is very similar. Other clinical and radiographic clues are used to narrow the diagnosis.
Clinical Features
editAmeloblastic fibroma is a rare benign mixed epithelial and mesenchymal odontogenic tumour as it contributes to approximately 2% of all odontogenic tumours. It often occurs in the first or second decade of life. Ameloblastic fibroma usually appears as painless swelling of the jaw in the posterior region of the mandible.[2] It can be associated with an impacted tooth[3] and it might impede eruption of other teeth. The lesion can be asymptomatic when it is small and most are incidental findings of routine dental radiographic imaging, etc.[3][4]
Diagnosis
editRadiographic Features
editRadiographically, ameloblastic fibroma has a variable appearance. It can appear as a unilocular lesion with smooth, well-defined margin when smaller. However, it can be multilocular when it is enlarged. It can be mistaken as dentigerous cyst as the lesion is often associated with an impacted tooth.[1][4]
Histopathology
editThe histopathology of ameloblastic fibromas resembles the stages of normal tooth development.[5] These mixed lesions consist of histologically distinct epithelial and mesenchymal tissues.[6] The epithelial tissue resembles dental lamina and enamel organ tissues, while the mesenchyme resembles the dental papilla.[7]
The epithelial component features strands which branch and join, or anastomose. This results in knots of differing mass, resembling islands in a loose stroma.[7] Cells in the strands tend to be cuboidal, but where budding occurs from the strands the knots resemble tooth caps. The bud-cap stage of normal development shows ameloblasts forming palisades of columnar cells adjacent to a starry-like, loosely formed layer known as stellate reticulum.[8] However, the pattern of budding strands is distinctive from normal development.[7] The Ameloblastic Fibroma epithelial tissue could be confused with the most common odontogenic tumour, the Ameloblastoma. Therefore the mesenchymal component is histologically important in differential diagnosis.[7]
The mesenchymal stroma in normal development is a rich myxoid connective tissue. It gives rise to the dental follicle which encapsulates the developing tooth.[8] In Ameloblastoma the stroma is mature, often fibrous. This is distinct from the mesenchymal element of Ameloblastic Fibroma which is devoid of collagen.[2] The Ameloblastic Fibroma stroma remains primitive, undifferentiated, cell-rich and myxoid.[7] Rarely, it may contain granular cells. However, this may also be observed in a hyperplastic dental follicle, and therefore other histological and radiological diagnostic features should be observed.[9]
The malignant ameloblastic fibroma will have features of malignant transformation such as mitotic figures in either epithelial or mesenchymal tissue.[8] There is a resemblance to fibrosarcoma. The malignant Ameloblastic Fibroma histologically shows transformation in the mesenchymal component with increased cellularity, accompanied by a progressive reduction in epithelial tissue.[1]
Classification
editAn ameloblastic fibroma is classified by The World Health Organisation as a benign mixed odontogenic tumour.[2] It develops from the dental tissues that grow into teeth. During human development, embryonic cells of ectoderm and mesenchyme produce epithelial and ectomesenchyme tissues. These proliferate and mature into ameloblasts and fibrous connective tissue,[4] and ultimately teeth. Ameloblastic fibromas contain both of these tissues, and its name is derived from them. It is a neoplasm, meaning it is a mass of abnormal growth of cells or tissue. If the mass contains hard dental tissues they are known as odontoma, which are not true neoplasm, but classified as hamartomatous lesions.[1]
Treatments
editAs ameloblastic fibromas are rare and the literature regarding treatment is limited there is controversy regarding treatment approach. A conservative treatment strategy, such as enucleation and curettage, is usually sufficient for small ameloblastic fibromas. However, extensive and aggressive lesions may require radical treatment such as in older patients who have likely high recurrence tendency.
If the ameloblastic fibroma is small, ‘reconstruction’ will not be required. Conservative treatment usually involves enucleation and thorough curettage of the affected area alongside extraction of the affected teeth.[1] Enucleation is the removal of an organ or tumor in such a way that it comes out clean and whole.[9] Thorough enucleation is important as there are reports of a high recurrence rate (Trodahl reported a 36.4% recurrence rate).[5] Immediate reconstruction is required post enucleation and curettage of the affected area.[6] Some patients may require reconstruction which can include a full thickness bone graft. This treatment allows the patient to retain oral function and as much facial structure as possible.
Occasionally, more radical treatment is required with excision of the tumour with a margin of healthy tissue.[8]
This kind of treatment also requires reconstruction of the affected area, with bone grafts often being the preferred choice for remodelling. This is done when the tumour is large or is deemed to have a high chance of malignant transformation.[7] In some cases the fibroma may envelop a nerve and may have to be removed too.[10]
Implants in compromised areas filled with a bone graft can prove useful for functional and aesthetic stability. Implant retained prosthesis can be placed and can make a vital overall functional and masticatory difference.[11]
Close radiographic and clinical follow up is important to identify recurrence and malignant transformation.[10]
Epidemiology
editOdontogenic tumours are uncommon, with a prevalence of around 2%[9] and only 1-2% of these are ameloblastic fibromas.[5] As they are rare, there is limited evidence available, mostly case studies. There is a slight male predilection, developing most commonly within the first two decades of life. They are often identified when tooth development is complete with the posterior mandible being the most common site. Although benign, ameloblastic fibromas that occur in later decades as well as a third of treated ameloblastic fibromas can recur and around 11% may undergo malignant transformation, though this figure is questioned.[1][6] Odontoma are the most prevalent of the odontogenic tumours in the early decades.[citation needed]
See also
editReferences
edit- ^ a b c d e f g h i j k l Nelson, Brenda L.; Folk, Gretchen S. (March 2009). "Ameloblastic Fibroma". Head and Neck Pathology. 3 (1): 51–53. doi:10.1007/s12105-008-0091-0. ISSN 1936-055X. PMC 2807540. PMID 20596990.
- ^ a b c Ponnam, SrinivasRao; Srivastava, Gautam; Smitha, B (2012). "Ameloblastic fibroma". Journal of Oral and Maxillofacial Pathology. 16 (3): 444–5. doi:10.4103/0973-029X.102515. ISSN 0973-029X. PMC 3519228. PMID 23248485.
- ^ a b Ealla, Kranti Kiran Reddy; Basavanapalli, Vijayabaskar Reddy; Velidandla, Surekha Reddy; Manikya, Sangameshwar; Ragulakollu, Rajesh; Danappanavar, Prasanna M.; Vennila, Vijayasree (2015). "Ameloblastic Fibroma of the Maxilla with Bilateral Presentation: Report of a Rare Case with Review of the Literature". Case Reports in Pediatrics. 2015: 250713. doi:10.1155/2015/250713. PMC 4299785. PMID 25628911.
- ^ a b c Odell, E. W. (2017). Cawson's essentials of oral pathology and oral medicine. Preceded by (work): Cawson, R. A. (Ninth ed.). [Edinburgh]. ISBN 978-0-7020-4982-8. OCLC 960030340.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ a b c Tozoglu, S.; Hatipoglu, M.; Aytekin, Z.; Gurer, E. I. (2016). "Europe PMC". European Journal of Dentistry. 10 (1): 139–143. doi:10.4103/1305-7456.175700. PMC 4784144. PMID 27011753.
- ^ a b c Vasconcelos, Belmiro C. E.; Andrade, Emanuel S. S.; Rocha, Nelson S.; Morais, Hécio H. A.; Carvalho, Ricardo W. F. (June 2009). "Treatment of large ameloblastic fibroma: a case report". Journal of Oral Science. 51 (2): 293–296. doi:10.2334/josnusd.51.293. ISSN 1880-4926. PMID 19550100.
- ^ a b c d e f Baris Bingül, Kenan (2017-08-24), Review of 101 benign epithelial, mesencymal and mixed odontogenic tumours, doi:10.26226/morressier.596dfd58d462b80292387c8c
- ^ a b c d Chrcanovic, Bruno Ramos; Brennan, Peter A.; Rahimi, Siavash; Gomez, Ricardo Santiago (April 2018). "Ameloblastic fibroma and ameloblastic fibrosarcoma: A systematic review". Journal of Oral Pathology & Medicine. 47 (4): 315–325. doi:10.1111/jop.12622. hdl:2043/23318. ISSN 1600-0714. PMID 28776760. S2CID 4873133.
- ^ a b c "NCI Dictionary of Cancer Terms". National Cancer Institute. 2011-02-02. Retrieved 2020-03-05.
- ^ a b Carroll, Conor; Gill, Mishaal; Bowden, Eleanor; O’Connell, John Ed; Shukla, Rajeev; Sweet, Chris (2019). "Ameloblastic Fibroma of the Mandible Reconstructed with Autogenous Parietal Bone: Report of a Case and Literature Review". Case Reports in Dentistry. 2019: 5149219. doi:10.1155/2019/5149219. PMC 6604494. PMID 31316839.
- ^ Mishra, Niraj; Pal, Umashankar (2012). "Placement of implants in an ossifying fibroma defect obliterated with demineralized, freeze-dried bone allograft and Plasma-rich growth factor". Contemporary Clinical Dentistry. 3 (4): 471–4. doi:10.4103/0976-237X.107444. ISSN 0976-237X. PMC 3636830. PMID 23633812.
Further reading
edit- Kahn, Michael A. Basic Oral and Maxillofacial Pathology. Volume 1. 2001.
- Wright, John M.; Vered, Marilena (February 28, 2017). "Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Odontogenic and Maxillofacial Bone Tumors". Head and Neck Pathology. 11 (1). Springer Science and Business Media LLC: 68–77. doi:10.1007/s12105-017-0794-1. ISSN 1936-055X. PMC 5340735. PMID 28247226.
- Crilly, M. (2013). "Pocketbook of oral disease". British Dental Journal. 214 (5). Springer Science and Business Media LLC: 267–268. doi:10.1038/sj.bdj.2013.266. ISSN 0007-0610.
- Miller, N. (2012). "Ten Cate's oral histology, 8th edition". British Dental Journal. 213 (4). Springer Science and Business Media LLC: 194. doi:10.1038/sj.bdj.2012.772. ISSN 0007-0610.
- Chen, Yan; Wang, Jing-Ming; Li, Tie-Jun (2007). "Ameloblastic fibroma: A review of published studies with special reference to its nature and biological behavior". Oral Oncology. 43 (10). Elsevier BV: 960–969. doi:10.1016/j.oraloncology.2007.05.009. ISSN 1368-8375.
- El-Naggar, Adel K.; Chan, John K. C.; Rubin Grandis, Jennifer; Takata, Takashi; Slootweg, Pieter Johannes; International Agency for Research on Cancer (2017). WHO classification of head and neck tumours. Lyon, France: International Agency for Research on Cancer (IARC). ISBN 978-92-832-2438-9. OCLC 990147303.
- Buchner, Amos; Merrell, Phillip W.; Carpenter, William M. (2006). "Relative Frequency of Central Odontogenic Tumors: A Study of 1,088 Cases from Northern California and Comparison to Studies from Other Parts of the World". Journal of Oral and Maxillofacial Surgery. 64 (9). Elsevier BV: 1343–1352. doi:10.1016/j.joms.2006.05.019. ISSN 0278-2391.
- Buchner, Amos; Vered, Marilena (2013). "Ameloblastic fibroma: a stage in the development of a hamartomatous odontoma or a true neoplasm? Critical analysis of 162 previously reported cases plus 10 new cases". Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 116 (5). Elsevier BV: 598–606. doi:10.1016/j.oooo.2013.06.039. ISSN 2212-4403.
- Slootweg, Pieter (2015). Pathology of the Maxillofacial Bones. Cham: Springer International Publishing. doi:10.1007/978-3-319-16961-3. ISBN 978-3-319-16960-6.