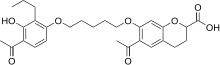
Ablukast (INN) is an experimental drug that is a leukotriene antagonist. It was investigated for potential applications in the treatment of inflammatory conditions, including asthma, skin disorders, and inflammatory bowel disease.[1][2] It reached Phase III clinical trials, but development was discontinued in 1996.[2]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H34O8 |
| Molar mass | 498.572 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
References
edit- ^ Rosenbach T, Csatò M, Czarnetzki BM (January 1988). "Studies on the role of leukotrienes in murine allergic and irritant contact dermatitis". The British Journal of Dermatology. 118 (1): 1–6. doi:10.1111/j.1365-2133.1988.tb01743.x. PMID 2829957. S2CID 1164113.
- ^ a b "Ablukast". Adis Insight.
External links
edit- Media related to Ablukast at Wikimedia Commons