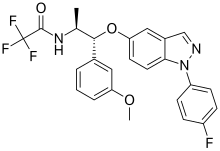
AZD-5423 is a nonsteroidal glucocorticoid and phase II experimental drug being developed by AstraZeneca[1] and disclosed at the spring 2013 American Chemical Society meeting in New Orleans to treat respiratory diseases and in particular chronic obstructive pulmonary disease.[2][3][4][5][6]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.205.965 |
| Chemical and physical data | |
| Formula | C25H21F4N3O3 |
| Molar mass | 487.455 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
It has completed a phase II clinical trial.[7]
See also
editReferences
edit- ^ Hemmerling M, Nilsson S, Edman K, Eirefelt S, Russell W, Hendrickx R, et al. (October 2017). "Selective Nonsteroidal Glucocorticoid Receptor Modulators for the Inhaled Treatment of Pulmonary Diseases". Journal of Medicinal Chemistry. 60 (20): 8591–8605. doi:10.1021/acs.jmedchem.7b01215. PMID 28937774.
- ^ "AZD 5423 - AdisInsight".
- ^ Drahl C (2013). "Liveblogging First-Time Disclosures of Drug Structures from #ACSNOLA".
- ^ GB2010051905 Combinations comprising a glucocorticoid receptor modulator for the treatment of respiratory diseases
- ^ SE2009050900 A combination of (a) glucocorticoid receptor modulator and (b) a muscarinic antagonist
- ^ SE2009000264 Combination of (a) glucocorticoid receptor modulator and (b) a β2-agonist
- ^ Clinical trial number NCT01555099 for "Multi-centre Study to Assess the Efficacy and Safety of AZD5423 in COPD Patients on a Background Therapy of Formoterol" at ClinicalTrials.gov
External links
edit