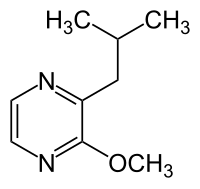
3-Isobutyl-2-methoxypyrazine (2-isobutyl-3-methoxypyrazine[1] also known as Grindstaff pyrazine) is a methoxypyrazine that is very similar to isopropyl methoxy pyrazine except that the alkyl side-group contains an isobutyl group attached to the carbon alpha to the methoxy sidegroup instead of an isopropyl side-group at that same carbon position.
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methoxy-3-(2-methylpropyl)pyrazine | |
| Other names
2-Isobutyl-3-methoxypyrazine; IBMP
Grindstaff pyrazine Bell pepper pyrazine | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.042.169 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H14N2O | |
| Molar mass | 166.224 g·mol−1 |
| Appearance | Liquid |
| Hazards | |
| Flash point | 80 °C (176 °F; 353 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Natural occurrence
editThe molecule is produced by some plants and is a contributor to the aroma of several plant-based foods.[2][3] It is also seen to be produced in some forms of blue-green algae;[4] however, it is most frequently found in members of the genus Capsicum. Specifically, it is what gives a bell pepper, along with other peppers, their distinctive smell.[5] This is to such an extent that Grindstaff pyrazine is sometimes just called the "bell pepper pyrazine." The human nose can detect concentrations of this molecule below the part per trillion level making it one of the most odor intensive compounds on earth.[6] Unlike capsaicin, which is produced and mainly stored in the placenta of the pepper, Grindstaff pyrazine is mainly contained in the flesh of the pepper.[7] This is why dried pepper flakes and other spices derived from the seeds and placenta of peppers lack the aroma of what is thought of as a "fresh pepper smell".
Uses
editIllicit drug prevention
edit3-isobutyl-2-methoxypyrazine has been noted, along with a few other food flavor additives, as a possible means of preventing the use of pseudoephedrine in the production of methamphetamine.[8] A number of pharmaceutical companies have proposed various physical means of deterring such illicit uses namely by altering the method of drug delivery. However, these physical methods only make it more difficult to extract the pseudophedrine and do nothing to prevent the chemical transformation to methamphetamine.[9] The addition of 3-isobutyl-2-methoxypyrazine to pseudophedrine effectively blocks the chemical production of methamphetamine from pseudophedrine while remaining biologically inert.
Fragrance and flavor
editSince 3-isobutyl-2-methoxypyrazine has such a powerful scent, it is often used in fragrances and cleaners. It poses little threat to children or pets, making its use in detergents, candles, deodorants, gums and candies quite widespread. Even though it is not very volatile, it is often considered a top note in perfumery because the fragrance can be detected at such low levels.[5] Chemically it behaves like a base note, since it has a low volatility; however, its pungency allows it to be identified with the other top notes in the fragrance even if its relative concentration in the air is much lower.[10]
References
edit- ^ 2-Isobutyl-3-methoxypyrazine in Sigma-Aldrich Catalogue
- ^ Kaneko, S.; Sakai, R.; Kumazawa, K.; Usuki, M.; Nishimura, O. (2013). "Key aroma compounds in roasted in-shell peanuts". Bioscience, Biotechnology, and Biochemistry. 77 (7): 1467–1473. doi:10.1271/bbb.130112. PMID 23832337.
- ^ Koch, A.; Doyle, C. L.; Matthews, M. A.; Williams, L. E.; Ebeler, S. E. (2010). "2-Methoxy-3-isobutylpyrazine in grape berries and its dependence on genotype". Phytochemistry. 71 (17–18): 2190–2198. Bibcode:2010PChem..71.2190K. doi:10.1016/j.phytochem.2010.09.006. PMID 20965529.
- ^ Peter, Andreas; Köster, Oliver; Schildknecht, Andrea; von Gunten, Urs (2009-05-01). "Occurrence of dissolved and particle-bound taste and odor compounds in Swiss lake waters". Water Research. 43 (8): 2191–2200. Bibcode:2009WatRe..43.2191P. doi:10.1016/j.watres.2009.02.016. PMID 19303129.
- ^ a b Roth, Klaus (2014). "The Biochemistry of Peppers". ChemViews. doi:10.1002/chemv.201400031.
- ^ Roth, Klaus (2009-04-01). "Allerlei vom Frühstücksei. Eine Oologisch-chemische Osterbetrachtung". Chemie in unserer Zeit. 43 (2): 100–114. doi:10.1002/ciuz.200900485. ISSN 1521-3781.
- ^ Camara, Bilal; Monéger, René (1978-01-01). "Free and esterified carotenoids in green and red fruits of Capsicum annuum". Phytochemistry. 17 (1): 91–93. Bibcode:1978PChem..17...91C. doi:10.1016/S0031-9422(00)89686-7.
- ^ Rajagopalan, Raghavan (March 31, 2016). "Prevention of Illicit Manufacture of Methamphetamine from Pseudoephedrine Using Food Flavor Excipients". United States Patent Application WO/2015/048597.
- ^ Skinner, H.F. (1990). "Methamphetamine synthesis via hydriodic acid/red phosphorus reduction of ephedrine". Forensic Science International. 48 (2): 123–134. doi:10.1016/0379-0738(90)90104-7.
- ^ Adams, T. B.; et al. (2002). "The FEMA GRAS assessment of pyrazine derivatives used as flavor ingredients". Food and Chemical Toxicology. 40 (4): 429–451. doi:10.1016/s0278-6915(01)00123-5. PMID 11893403.
- ^ Lautenschlager, C.; Leal, W. S.; Clardy, J. (2007). "Bombyx mori Pheromone-Binding Protein Binding Nonpheromone Ligands: Implications for Pheromone Recognition". Structure. 15 (9): 1148–1154. doi:10.1016/j.str.2007.07.013. PMC 2072049. PMID 17850754.