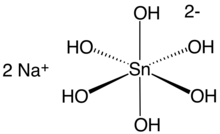
Sodium stannate, formally sodium hexahydroxostannate(IV), is the inorganic compound with the formula Na2[Sn(OH)6]. This colourless salt forms upon dissolving metallic tin or tin(IV) oxide in sodium hydroxide and is used as a stabiliser for hydrogen peroxide.[2] In older literature, stannates are sometimes represented as having the simple oxyanion SnO32−,[3] in which case this compound is sometimes named as sodium stannate–3–water and represented as Na2SnO3·3H2O, a hydrate with three waters of crystallisation.[1] The anhydrous form of sodium stannate, Na2SnO3, is recognised as a distinct compound with its own CAS Registry Number,[4] 12058-66-1 , and a distinct material safety data sheet.[5]
| |
| Names | |
|---|---|
| IUPAC name
Sodium hexahydroxostannate(IV)
| |
| Other names
disodium hexahydroxyltin
Sodium stannate(IV) sodium stannate–3–water sodium tin(IV) oxide hydrate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.554 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H6Na2O6Sn | |
| Molar mass | 266.73 g/mol |
| Appearance | Colorless or white solid |
| Density | 4.68 g/cm3 |
| Boiling point | N/A |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H314, H315, H319, H335, H412 | |
| P260, P261, P264, P271, P273, P280, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 57 °C (135 °F; 330 K) |
| N/A | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2132 mg/kg [Mouse] |
| Safety data sheet (SDS) | [1][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Alkali metal stannate compounds are prepared by dissolving elemental tin in a suitable metal hydroxide, in the case of sodium stannate by the reaction:[6]
- Sn + 2 NaOH + 4 H2O → Na2[Sn(OH)6] + 2 H2
A similar reaction occurs when tin dioxide is dissolved in base:
- SnO2 + 2 NaOH + 2 H2O → Na2[Sn(OH)6]
The anhydrous form can also be prepared from tin dioxide by roasting with sodium carbonate in a mixed carbon monoxide / carbon dioxide environment:[7]
- SnO2 + Na2CO3 → Na2SnO3 + CO2
The anion is a coordination complex that is octahedral in shape, similar to most stannates, such as the hexachlorostannate anion [SnCl6]2−. The Sn—O bond distances average 2.071 Å.[8]
See also
editReferences
edit- ^ a b "Material Safety Data Sheet – sodium stannate trihydrate MSDS". Science Lab. 21 May 2013. Archived from the original on 1 June 2012. Retrieved 1 June 2017.
- ^ Clark, John D. (1972). Ignition! An Informal History of Liquid Rocket Propellants. Rutgers University Press. ISBN 0813507251.
- ^ Similarly, stannites are sometimes represented with the anion SnO22−
- ^ National Center for Biotechnology Information (2017). "Sodium Stannate". PubChem. Retrieved 1 June 2017.
- ^ "Sodium Stannate MSDS" (PDF). Santa Cruz Biotechnology. 14 June 2011. Retrieved 1 June 2017.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0750633654.
- ^ Zhang, Yuanbo; Su, Zijian; Liu, Bingbing; You, Zhixiong; Yang, Guang; Li, Guanghui; Jiang, Tao (2014). "Sodium stannate preparation from stannic oxide by a novel soda roasting–leaching process". Hydrometallurgy. 146: 82–88. Bibcode:2014HydMe.146...82Z. doi:10.1016/j.hydromet.2014.03.008.
- ^ Jacobs, Herbert; Stahl, Rainer (2000). "Neubestimmung der Kristallstrukturen der Hexahydroxometallate Na2Sn(OH)6, K2Sn(OH)6 und K2Pb(OH)6". Z. Anorg. Allg. Chem. (in German). 626 (9): 1863–1866. doi:10.1002/1521-3749(200009)626:9<1863::AID-ZAAC1863>3.0.CO;2-M.
