Müllerian agenesis, also known as Müllerian aplasia, vaginal agenesis, or Mayer–Rokitansky–Küster–Hauser syndrome (MRKH syndrome), is a congenital malformation characterized by a failure of the Müllerian ducts to develop, resulting in a missing uterus and variable degrees of vaginal hypoplasia of its upper portion. Müllerian agenesis (including absence of the uterus, cervix and/or vagina) is the cause in 15% of cases of primary amenorrhoea.[2] Because most of the vagina does not develop from the Müllerian duct, instead developing from the urogenital sinus, along with the bladder and urethra, it is present even when the Müllerian duct is completely absent. Because ovaries do not develop from the Müllerian ducts, affected people might have normal secondary sexual characteristics but are infertile due to the lack of a functional uterus. However, biological motherhood is possible through uterus transplantation or use of gestational surrogates.
| Müllerian agenesis | |
|---|---|
| Other names |
|
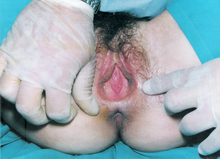 | |
| A patient displaying Müllerian agenesis | |
| Specialty | Gynecology |
| Symptoms | Missing uterus and variable degrees of vaginal hypoplasia |
| Frequency | 1 in 4,500 females[1] |
Müllerian agenesis is hypothesized to be a result of autosomal dominant inheritance with incomplete penetrance and variable expressivity, which contributes to the complexity involved in identifying the underlying causal mechanisms. Because of the variance in inheritance, penetrance and expressivity patterns, Müllerian agenesis is subdivided into two types: type 1, in which only the structures developing from the Müllerian duct are affected (the upper vagina, cervix, and uterus), and type 2, wherein the same structures are affected, but other body systems, most often the renal and skeletal systems, have additional malformations. Type 2 includes MURCS (Müllerian Renal Cervical Somite).
The majority of Müllerian agenesis cases are characterized as sporadic, but familial cases have provided evidence that, at least for some patients, it is an inherited disorder. The underlying causes are still being investigated, but several causative genes have been studied for their possible association with the syndrome. Most of these studies have served to rule-out genes as causative factors, but thus far, only WNT4 has been associated with Müllerian agenesis with hyperandrogenism.[3][4]
Reports of Müllerian agenesis can be traced back to Hippocrates (460 B.C.–377 B.C.).[5][6] The medical eponym honors August Franz Josef Karl Mayer (1787–1865), Carl Freiherr von Rokitansky (1804–1878), Hermann Küster (1879–1964) and Georges Andre Hauser (1921–2009).
Signs and symptoms
editA female with this condition is hormonally normal; that is, the woman will enter puberty with development of secondary sexual characteristics including thelarche (breast development) and pubarche (pubic hair). The woman's karyotype will be 46,XX. At least one ovary is intact, if not both, and ovulation usually occurs. Typically, the vagina is shortened and intercourse may, in some cases, be difficult and painful. Medical examination supported by gynecologic ultrasonography demonstrates a complete or partial absence of the cervix, uterus, and vagina.[citation needed]
If there is no uterus, a woman with Müllerian agenesis cannot carry a pregnancy without intervention. It is possible for the woman to have genetic offspring by in vitro fertilization (IVF) and surrogacy. Successful uterine transplant has been performed in limited numbers of patients, resulting in several live births, but the technique is not widespread or accessible to many women.[7]
A woman with Müllerian agenesis typically discovers the condition when, during puberty years, the menstrual cycle does not start (primary amenorrhoea). Some find out earlier through surgeries for other conditions, such as a hernia.[citation needed]
Causes
editThe etiology of Müllerian agenesis in many cases remains elusive.[8] However, mutations in a variety of different genes have been implicated in causing MRKH syndrome.[9][10][11] The typical and atypical forms of the disorder are presumably caused by mutations in different genes.[8]
WNT4 (found on the short arm (p) of chromosome 1) has been clearly implicated in the atypical version of this disorder. A genetic mutation causes a leucine to proline residue substitution at amino acid position 12.[12] This occurrence reduces the intranuclear levels of β catenin. In addition, it removes the inhibition of steroidogenic enzymes like 3β-hydroxysteriod dehydrogenase and 17α-hydroxylase. Patients therefore have androgen excess.[12] Furthermore, without WNT4, the Müllerian duct is either deformed or absent. Female reproductive organs, such as the cervix, fallopian tubes, and much of the vagina, are hence affected.[13]
An association with 17q12 microdeletion syndrome, a deletion mutation in the long arm (q) of chromosome 17, has been reported. The gene LHX1 is located in this region and may be the cause of a number of these cases.[14]
Diagnosis
editClassification
edit- Typical Müllerian agenesis – Isolated uterovaginal aplasia/hypoplasia
- Prevalence – 64%
- Atypical Müllerian agenesis – Uterovaginal aplasia/hypoplasia with renal malformation or uterovaginal aplasia/hypoplasia with ovarian dysfunction
- Prevalence – 24%
- MURCS syndrome – Uterovaginal aplasia/hypoplasia with renal malformation, skeletal malformation, and cardiac malformation
- Prevalence – 12%[12]
Treatment
editA number of treatments have become available to create a functioning vagina. Standard approaches use vaginal dilators and/or surgery to develop a functioning vagina to allow for penetrative sexual intercourse.
A number of surgical approaches have been used. In the McIndoe procedure, a skin graft is applied to form an artificial vagina. After the surgery, dilators are still necessary to prevent vaginal stenosis. The Vecchietti procedure has been shown to result in a vagina that is comparable to a typical vagina.[15][16] In the Vecchietti procedure, a small plastic “olive” is threaded against the vaginal area, and the threads are drawn through the vaginal skin, up through the abdomen and navel using laparoscopic surgery. There, the threads are attached to a traction device. The traction device is then tightened daily so the “olive” is pulled inwards and stretches the vagina by approximately 1 cm per day, creating a vagina approximately 7 cm deep in 7 days. The operation takes approximately 45 minutes. [17] Another approach is the use of an autotransplant of a resected sigmoid colon using laparoscopic surgery; results are reported to be very good, with the transplant becoming a functional vagina.[18]
Uterine transplantation has been performed in a number of people with Müllerian agenesis, but the surgery is still in the experimental stage.[19] Since ovaries are present, people with this condition can have genetic children through IVF with embryo transfer to a gestational carrier. Some also choose to adopt.[20][21]
Experimental
editIn October 2014, it was reported that a month earlier a 36-year-old Swedish woman became the first woman with a transplanted uterus to give birth to a healthy baby. The mother was born without a uterus, but had functioning ovaries. She and the father used IVF to produce 11 embryos, which were then frozen. Doctors at the University of Gothenburg then performed the uterus transplant, the donor being a 61-year-old family friend. One of the frozen embryos was implanted a year after the transplant, and the baby boy was born prematurely at 31 weeks after the mother developed pre-eclampsia.[22]
As of 2023 more than 100 womb transplants have taken place with around 50 babies have been born worldwide[23][24]
Laboratory-grown vagina
editPromising research includes the use of laboratory-grown structures, which are less subject to the complications of non-vaginal tissue, and may be grown using the woman's own cells as a culture source.[25][26]
A 2014 study and experiment with laboratory-grown engineered vaginas using the patient's own cells resulted in fully functional vaginas capable of menstruation, sustaining penetrative sex, and orgasm in 4 patients, showing promise of fully correcting this condition.[27][28]
Epidemiology
editThe prevalence remains sparsely investigated. To date, two population-based nationwide studies have been conducted both estimating a prevalence about 1 in 5,000 live female births.[contradictory][29][30] According to some reports, Queen Amalia of Greece may have had the syndrome, but a 2011 review of the historical evidence concludes that it is not possible to determine the inability of her and her husband to have a child.[31][6] Their inability to conceive an heir contributed to the overthrow of the king King Otto.[31]
People with Müllerian agenesis
edit- Ben Barres, transgender male scientist[32]
- Shon Klose, Australian intersex activist and musician[33][34]
- Esther Morris Leidolf, medical sociologist and intersex activist
- Molly McGlynn, Canadian film director who made the 2023 film Fitting In about her experiences being diagnosed with the condition[35]
- Jaclyn Schultz, Miss Michigan[36]
Suspected cases
edit- Amalia of Oldenburg, Queen of Greece[37]
See also
editReferences
edit- ^ "Mayer–Rokitansky–Küster–Hauser syndrome: Diagnosis, Management, and Treatment". ACOG. January 2018. Retrieved 21 January 2018.
- ^ Welt CK, Barbieri RL. "Etiology, diagnosis, and treatment of primary amenorrhea". Retrieved 19 November 2015.
- ^ Woten M. "Quick Lesson: Mayer–Rokitansky–Kuster–Hauser Syndrome". In Cinahl DP (ed.). Information Systems.
- ^ Fontana L, Gentilin B, Fedele L, Gervasini C, Miozzo M (February 2017). "Genetics of Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome". Clinical Genetics. 91 (2): 233–246. doi:10.1111/cge.12883. PMID 27716927. S2CID 283394.
- ^ Goldwyn RM (March 1977). "History of attempts to form a vagina". Plastic and Reconstructive Surgery. 59 (3): 319–29. doi:10.1097/00006534-197703000-00002. PMID 320610. S2CID 39727188.
- ^ a b Patnaik SS, Brazile B, Dandolu V, Ryan PL, Liao J (January 2015). "Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome: a historical perspective". Gene. 555 (1): 33–40. doi:10.1016/j.gene.2014.09.045. PMID 25260227.
- ^ Lewis T (10 July 2016). "Uterus transplants: My sister gave me her womb". The Guardian. Retrieved 10 July 2016.
- ^ a b Herlin MK, Petersen MB, Brännström M (August 2020). "Mayer–Rokitansky–Küster-Hauser (MRKH) syndrome: a comprehensive update". Orphanet J Rare Dis. 15 (1): 214. doi:10.1186/s13023-020-01491-9. PMC 7439721. PMID 32819397.
- ^ Ledig S, Wieacker P (2018). "Clinical and genetic aspects of Mayer–Rokitansky–Küster–Hauser syndrome". Med Genet. 30 (1): 3–11. doi:10.1007/s11825-018-0173-7. PMC 5838123. PMID 29527097.
- ^ Kyei Barffour I, Kyei Baah Kwarkoh R (March 2021). "GREB1L as a candidate gene of Mayer–Rokitansky–Küster–Hauser Syndrome". Eur J Med Genet. 64 (3): 104158. doi:10.1016/j.ejmg.2021.104158. PMID 33548512. S2CID 231875330.
- ^ Gatti M, Tolva G, Bergamaschi S, Giavoli C, Esposito S, Marchisio P, Milani D (October 2018). "Mayer–Rokitansky–Küster–Hauser Syndrome and 16p11.2 Recurrent Microdeletion: A Case Report and Review of the Literature". J Pediatr Adolesc Gynecol. 31 (5): 533–535. doi:10.1016/j.jpag.2018.04.003. PMID 29730431. S2CID 19145776.
- ^ a b c Sultan C, Biason-Lauber A, Philibert P (January 2009). "Mayer–Rokitansky–Kuster–Hauser syndrome: recent clinical and genetic findings". Gynecological Endocrinology. 25 (1): 8–11. doi:10.1080/09513590802288291. PMID 19165657. S2CID 33461252.
- ^ "WNT4 Müllerian aplasia and ovarian dysfunction". Genetics Home Reference. Archived from the original on 2014-06-14. Retrieved 2012-08-18.
- ^ Ledig S, Brucker S, Barresi G, Schomburg J, Rall K, Wieacker P (2012) Frame shift mutation of LHX1 is associated with Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome. Hum Reprod
- ^ Vecchietti G (1965). "[Creation of an artificial vagina in Rokitansky–Küster–Hauser syndrome]". Attualita di Ostetricia e Ginecologia (in Italian). 11 (2): 131–47. PMID 5319813.
- ^ Fedele L, Bianchi S, Tozzi L, Borruto F, Vignali M (November 1996). "A new laparoscopic procedure for creation of a neovagina in Mayer–Rokitansky–Kuster–Hauser syndrome". Fertility and Sterility. 66 (5): 854–7. doi:10.1016/S0015-0282(16)58653-1. PMID 8893702.
- ^ "Vecchietti Procedure" (PDF). University College University Hospitals. Archived from the original (PDF) on 2009-04-11. Retrieved 2010-04-03..
- ^ Hold MK (2007-01-16). "Modernes Management der angeborenen (Mayer–Rokitansky–Küster–Hauser, MRKH-Syndrom) und erworbenen Vaginalaplasie" (PDF). Frauenheilkunde-Aktuell (in German).
- ^ Ozkan O, Akar ME, Ozkan O, Erdogan O, Hadimioglu N, Yilmaz M, Gunseren F, Cincik M, Pestereli E, Kocak H, Mutlu D, Dinckan A, Gecici O, Bektas G, Suleymanlar G (February 2013). "Preliminary results of the first human uterus transplantation from a multiorgan donor". Fertility and Sterility. 99 (2): 470–6. doi:10.1016/j.fertnstert.2012.09.035. PMID 23084266.
- ^ Akhter N, Begum B (3 February 2013). "Evaluation and management of cases of primary amenorrhoea with MRKH syndrome". Bangladesh Medical Journal Khulna. 45 (1–2): 24–29. doi:10.3329/bmjk.v45i1-2.13626.
- ^ "Rokitansky Syndrome: Information for Parents / Carers" (PDF). Saint Mary's Hospital, Manchester. Archived from the original (PDF) on 19 February 2017. Retrieved 11 April 2014.
- ^ "First womb-transplant baby born". BBC News. 2014-10-04. Retrieved 2023-08-27.
- ^ Brännström, Mats; Racowsky, Catherine; Richards, Elliott G.; Flyckt, Rebecca; Stillman, Robert J.; O’Brien, Jeanne E.; Ryan, Ginny L.; de Ziegler, Dominique (June 2023). "Absolute uterine infertility a cornelian dilemma: uterine transplantation or surrogacy?". Fertility and Sterility. 119 (6): 918–929. doi:10.1016/j.fertnstert.2023.04.005. PMID 37037300.
- ^ "Woman receives sister's womb in first UK transplant". BBC News. 2023-08-22. Retrieved 2023-08-27.
- ^ "Laboratory-grown vaginas implanted in patients". Retrieved 14 April 2014.
- ^ Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, López-Bayghen E, Valencia P, Ordorica-Flores R, Soker S, Yoo JJ, Atala A (July 2014). "Tissue-engineered autologous vaginal organs in patients: a pilot cohort study". Lancet. 384 (9940): 329–36. doi:10.1016/S0140-6736(14)60542-0. PMID 24726478. S2CID 6296110.
- ^ Catherine de Lange (2014). "Engineered vaginas grown in women for the first time". New Scientist. 222 (2965): 10. Bibcode:2014NewSc.222...10D. doi:10.1016/S0262-4079(14)60758-2.
- ^ Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, López-Bayghen E, Valencia P, Ordorica-Flores R, Soker S, Yoo JJ, Atala A (July 2014). "Tissue-engineered autologous vaginal organs in patients: a pilot cohort study". Lancet. 384 (9940): 329–36. doi:10.1016/S0140-6736(14)60542-0. PMID 24726478. S2CID 6296110.
- ^ Aittomäki K, Eroila H, Kajanoja P (September 2001). "A population-based study of the incidence of Müllerian aplasia in Finland". Fertility and Sterility. 76 (3): 624–5. doi:10.1016/s0015-0282(01)01963-x. PMID 11570363.
- ^ Herlin M, Bjørn AM, Rasmussen M, Trolle B, Petersen MB (October 2016). "Prevalence and patient characteristics of Mayer–Rokitansky–Küster–Hauser syndrome: a nationwide registry-based study". Human Reproduction. 31 (10): 2384–90. doi:10.1093/humrep/dew220. PMID 27609979.
- ^ a b Poulakou-Rebelakou E, Tsiamis C, Tompros N, Creatsas G (March 2011). "The lack of a child, the loss of a throne: the infertility of the first royal couple of Greece (1833–62)". The Journal of the Royal College of Physicians of Edinburgh. 41 (1): 73–7. doi:10.4997/JRCPE.2011.115. PMID 21365071.
- ^ Morgan, Jules (2019). "Coming out as a male scientist in a man's world" (PDF). The Lancet. 7 (4): 275–9. doi:10.1016/j.neuron.2013.10.005. PMID 24139033.
- ^ sleath, emma (2014-12-03). "I am intersex: Shon Klose's story". ABC Alice Springs. Retrieved 2019-10-27.
- ^ One Plus One: Shon Klose, Australian Broadcasting Corporation, 2015-09-28, retrieved 2019-10-27
- ^ Chandler Levack, "TIFF 2023: The bold risks and bloody rewards of Canada’s Molly McGlynn" The Globe and Mail, September 5, 2023.
- ^ "Why This Woman Is Proud to Be Known as "The Pageant Queen Without a Uterus"". Cosmopolitan. 2015-01-20. Retrieved 2018-09-09.
- ^ "The infertility of the first royal couple of Greece" (PDF). Royal college of physicians of Edinburgh.
Further reading
edit- Morcel K, Camborieux L, Guerrier D (March 2007). "Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome". Orphanet Journal of Rare Diseases. 2 (13): 13. doi:10.1186/1750-1172-2-13. PMC 1832178. PMID 17359527.
- Varner RE, Younger JB, Blackwell RE (June 1985). "Müllerian dysgenesis". The Journal of Reproductive Medicine. 30 (6): 443–50. PMID 4020785.