Lecithin–cholesterol acyltransferase
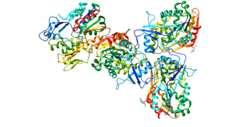
Lecithin–cholesterol acyltransferase (LCAT, also called phosphatidylcholine–sterol O-acyltransferase) is an enzyme found in many animals, including humans. It converts free cholesterol into cholesteryl ester, a more hydrophobic form of cholesterol. This process sequesters cholesterol ester into the core of a lipoprotein particle, eventually making the newly synthesized HDL spherical and forcing the reaction to become unidirectional since the particles are removed from the surface. The enzyme is bound to high-density lipoproteins (HDLs) (alpha-LCAT) and LDLs (beta-LCAT) in the blood plasma.[5] LCAT deficiency can cause impaired vision due to cholesterol corneal opacities, anemia, and kidney damage.[6] It belongs to the family of phospholipid:diacylglycerol acyltransferases.
Interactive pathway map
editClick on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "Statin_Pathway_WP430".
See also
editReferences
edit- ^ a b c GRCh38: Ensembl release 89: ENSG00000213398 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000035237 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Lecithin-Cholesterol Acyltransferase Deficiency: Overview, Presentation, Differential Diagnosis". 2016-08-08.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Reference, Genetics Home. "LCAT gene". Genetics Home Reference. Retrieved 2016-12-11.
Further reading
edit- Dobiásová M, Frohlich J (1999). "Advances in understanding of the role of lecithin cholesterol acyltransferase (LCAT) in cholesterol transport". Clin Chim Acta. 286 (1–2): 257–71. doi:10.1016/S0009-8981(99)00106-0. PMID 10511297.
- Kuivenhoven JA, Pritchard H, Hill J, et al. (1997). "The molecular pathology of lecithin:cholesterol acyltransferase (LCAT) deficiency syndromes". J. Lipid Res. 38 (2): 191–205. doi:10.1016/S0022-2275(20)37433-2. PMID 9162740.
- de Vries R, Borggreve SE, Dullaart RP (2004). "Role of lipases, lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein in abnormal high density lipoprotein metabolism in insulin resistance and type 2 diabetes mellitus". Clin. Lab. 49 (11–12): 601–13. PMID 14651331.
- Teisberg P, Gjone E, Olaisen B (1975). "Genetics of LCAT (lecithin: cholesterol acyltransferase) deficiency". Ann. Hum. Genet. 38 (3): 327–31. doi:10.1111/j.1469-1809.1975.tb00617.x. PMID 806250. S2CID 42785012.
- Cogan DG, Kruth HS, Datilis MB, Martin N (1993). "Corneal opacity in LCAT disease". Cornea. 11 (6): 595–9. doi:10.1097/00003226-199211000-00021. PMID 1468226.
- Skretting G, Blomhoff JP, Solheim J, Prydz H (1992). "The genetic defect of the original Norwegian lecithin:cholesterol acyltransferase deficiency families". FEBS Lett. 309 (3): 307–10. doi:10.1016/0014-5793(92)80795-I. PMID 1516702. S2CID 26714265.
- Skretting G, Prydz H (1992). "An amino acid exchange in exon I of the human lecithin: cholesterol acyltransferase (LCAT) gene is associated with fish eye disease". Biochem. Biophys. Res. Commun. 182 (2): 583–7. doi:10.1016/0006-291X(92)91772-I. PMID 1571050.
- Furukawa Y, Urano T, Hida Y, et al. (1992). "Interaction of rat lecithin-cholesterol acyltransferase with rat apolipoprotein A-I and with lecithin-cholesterol vesicles". J. Biochem. 111 (3): 413–8. doi:10.1093/oxfordjournals.jbchem.a123771. PMID 1587806.
- Minnich A, Collet X, Roghani A, et al. (1992). "Site-directed mutagenesis and structure-function analysis of the human apolipoprotein A-I. Relation between lecithin-cholesterol acyltransferase activation and lipid binding". J. Biol. Chem. 267 (23): 16553–60. doi:10.1016/S0021-9258(18)42038-8. PMID 1644835.
- Bujo H, Kusunoki J, Ogasawara M, et al. (1992). "Molecular defect in familial lecithin:cholesterol acyltransferase (LCAT) deficiency: a single nucleotide insertion in LCAT gene causes a complete deficient type of the disease". Biochem. Biophys. Res. Commun. 181 (3): 933–40. doi:10.1016/0006-291X(91)92026-G. PMID 1662503.
- Gotoda T, Yamada N, Murase T, et al. (1991). "Differential phenotypic expression by three mutant alleles in familial lecithin:cholesterol acyltransferase deficiency". Lancet. 338 (8770): 778–81. doi:10.1016/0140-6736(91)90665-C. PMID 1681161. S2CID 9708282.
- Klein HG, Lohse P, Pritchard PH, et al. (1992). "Two different allelic mutations in the lecithin-cholesterol acyltransferase gene associated with the fish eye syndrome. Lecithin-cholesterol acyltransferase (Thr123----Ile) and lecithin-cholesterol acyltransferase (Thr347----Met)". J. Clin. Invest. 89 (2): 499–506. doi:10.1172/JCI115612. PMC 442879. PMID 1737840.
- Maeda E, Naka Y, Matozaki T, et al. (1991). "Lecithin-cholesterol acyltransferase (LCAT) deficiency with a missense mutation in exon 6 of the LCAT gene". Biochem. Biophys. Res. Commun. 178 (2): 460–6. doi:10.1016/0006-291X(91)90129-U. PMID 1859405.
- Funke H, von Eckardstein A, Pritchard PH, et al. (1991). "A molecular defect causing fish eye disease: an amino acid exchange in lecithin-cholesterol acyltransferase (LCAT) leads to the selective loss of alpha-LCAT activity". Proc. Natl. Acad. Sci. U.S.A. 88 (11): 4855–9. Bibcode:1991PNAS...88.4855F. doi:10.1073/pnas.88.11.4855. PMC 51765. PMID 2052566.
- Taramelli R, Pontoglio M, Candiani G, et al. (1990). "Lecithin cholesterol acyl transferase deficiency: molecular analysis of a mutated allele". Hum. Genet. 85 (2): 195–9. doi:10.1007/BF00193195. PMID 2370048. S2CID 23994746.
- Rogne S, Skretting G, Larsen F, et al. (1987). "The isolation and characterisation of a cDNA clone for human lecithin:cholesterol acyl transferase and its use to analyse the genes in patients with LCAT deficiency and fish eye disease". Biochem. Biophys. Res. Commun. 148 (1): 161–9. doi:10.1016/0006-291X(87)91090-4. PMID 2823801.
- Tata F, Chaves ME, Markham AF, et al. (1987). "The isolation and characterisation of cDNA and genomic clones for human lecithin: cholesterol acyltransferase". Biochim. Biophys. Acta. 910 (2): 142–8. doi:10.1016/0167-4781(87)90066-2. PMID 2823898.
- Yang CY, Manoogian D, Pao Q, et al. (1987). "Lecithin:cholesterol acyltransferase. Functional regions and a structural model of the enzyme". J. Biol. Chem. 262 (7): 3086–91. doi:10.1016/S0021-9258(18)61472-3. PMID 2880847.
- McLean J, Fielding C, Drayna D, et al. (1986). "Cloning and expression of human lecithin-cholesterol acyltransferase cDNA". Proc. Natl. Acad. Sci. U.S.A. 83 (8): 2335–9. Bibcode:1986PNAS...83.2335M. doi:10.1073/pnas.83.8.2335. PMC 323291. PMID 3458198.
- Azoulay M, Henry I, Tata F, et al. (1987). "The structural gene for lecithin:cholesterol acyl transferase (LCAT) maps to 16q22". Ann. Hum. Genet. 51 (Pt 2): 129–36. doi:10.1111/j.1469-1809.1987.tb01054.x. PMID 3674753. S2CID 31911235.
- McLean J, Wion K, Drayna D, et al. (1987). "Human lecithin-cholesterol acyltransferase gene: complete gene sequence and sites of expression". Nucleic Acids Res. 14 (23): 9397–406. doi:10.1093/nar/14.23.9397. PMC 311966. PMID 3797244.
External links
edit- Lecithin+Cholesterol+Acyltransferase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P04180 (Phosphatidylcholine-sterol acyltransferase) at the PDBe-KB.