Lanthanum monosulfide is a binary inorganic chemical compound of lanthanum metal and sulfur with the chemical formula LaS.[1][2]
| |
| Names | |
|---|---|
| IUPAC name
lanthanum; sulfide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| |
| |
| Properties | |
| LaS | |
| Molar mass | 170.97 g·mol−1 |
| Appearance | golden crystals |
| Density | 5.61 g/cm3 |
| Melting point | 2,300 °C (4,170 °F; 2,570 K) |
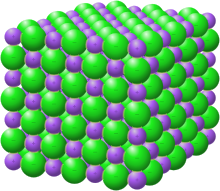
| Structure | |
| Cubic | |
| Related compounds | |
Related compounds
|
Samarium monosulfide |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
editLanthanum monosulfide can be prepared from the effect of sulfur vapor on metallic lanthanum:
- La + S → LaS
It can also be prepared from the reduction of La2S3 with metallic La.[3]
Physical properties
editLanthanum monosulfide forms golden crystals[4] of the cubic system, space group Fm3m, cell parameters a = 0.586 nm, Z = 4, structurally isomorphous with NaCl.[5]
References
edit- ^ Cater, E. D.; Lee, T. E.; Johnson, E. W.; Rauh, E. G.; Eick, H. A. (1965). "Vaporization, thermodynamics, and dissociation energy of lanthanum monosulfide". NIST. p. 2684. Retrieved 26 July 2024.
- ^ "Lanthanum Monosulfide". American Elements. Retrieved 26 July 2024.
- ^ Soviet Research on Complex and Coordination Compounds: Inorganic complexes. 1960. p. 579. Retrieved 26 July 2024.
- ^ Perry, Dale L. (19 April 2016). Handbook of Inorganic Compounds. CRC Press. p. 220. ISBN 978-1-4398-1462-8. Retrieved 26 July 2024.
- ^ Macintyre, Jane E. (23 July 1992). Dictionary of Inorganic Compounds. CRC Press. p. 3548. ISBN 978-0-412-30120-9. Retrieved 26 July 2024.