β-Lactam antibiotics (beta-lactam antibiotics) are antibiotics that contain a β-lactam ring in their chemical structure. This includes penicillin derivatives (penams), cephalosporins and cephamycins (cephems), monobactams, carbapenems[1] and carbacephems.[2] Most β-lactam antibiotics work by inhibiting cell wall biosynthesis in the bacterial organism and are the most widely used group of antibiotics. Until 2003, when measured by sales, more than half of all commercially available antibiotics in use were β-lactam compounds.[3] The first β-lactam antibiotic discovered, penicillin, was isolated from a strain of Penicillium rubens (named as Penicillium notatum at the time).[4][5]
| β-Lactam antibiotic | |
|---|---|
| Drug class | |
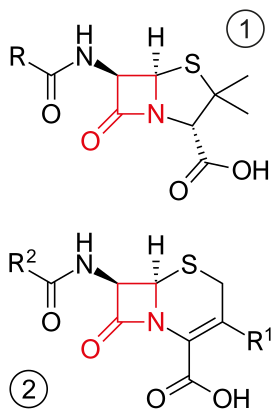 Core structure of penicillins (top) and cephalosporins (bottom) the 2 most common groups of β-lactam antibiotics . β-lactam ring in red. | |
| Class identifiers | |
| Use | Bacterial infection |
| ATC code | J01C |
| Biological target | Penicillin binding protein |
| External links | |
| MeSH | D047090 |
| Legal status | |
| In Wikidata | |
Bacteria often develop resistance to β-lactam antibiotics by synthesizing a β-lactamase, an enzyme that attacks the β-lactam ring. To overcome this resistance, β-lactam antibiotics can be given with β-lactamase inhibitors such as clavulanic acid.[6]
Medical use
editβ-Lactam antibiotics are indicated for the prevention and treatment of bacterial infections caused by susceptible organisms. At first, β-lactam antibiotics were mainly active only against gram-positive bacteria, yet the recent development of broad-spectrum β-lactam antibiotics active against various gram-negative organisms has increased their usefulness.[citation needed]
In uninflamed (normal) brain meninges, the penetration of beta-lactam antibiotics is low, at 0.15 of AUCCSF/AUCS ratio (the ratio of area under curve of cerebrosopinal fluid against area under curve of serum).[7]
Adverse effects
editAdverse drug reactions
editCommon adverse drug reactions for the β-lactam antibiotics include diarrhea, nausea, rash, urticaria, superinfection (including candidiasis).[8]
Infrequent adverse effects include fever, vomiting, erythema, dermatitis, angioedema, pseudomembranous colitis.[8]
Pain and inflammation at the injection site is also common for parenterally administered β-lactam antibiotics.[citation needed]
Allergy/hypersensitivity
editImmunologically mediated adverse reactions to any β-lactam antibiotic may occur in up to 10% of patients receiving that agent (a small fraction of which are truly IgE-mediated allergic reactions, see amoxicillin rash). Anaphylaxis will occur in approximately 0.01% of patients.[8][9] There is perhaps a 5–10% cross-sensitivity between penicillin-derivatives, cephalosporins, and carbapenems;[citation needed] but this figure has been challenged by various investigators.[who?][citation needed]
Nevertheless, the risk of cross-reactivity is sufficient to warrant the contraindication of all β-lactam antibiotics in patients with a history of severe allergic reactions (urticaria, anaphylaxis, interstitial nephritis) to any β-lactam antibiotic. Rarely, allergic reactions have been triggered by exposure from kissing and sexual contact with a partner who is taking these antibiotics.[10]
A Jarisch–Herxheimer reaction may occur after initial treatment of a spirochetal infection such as syphilis with a β-lactam antibiotic.[citation needed]
Mechanism of action
editInhibition of cell wall synthesis
editβ-Lactam antibiotics are bactericidal, and act by inhibiting the synthesis of the peptidoglycan layer of bacterial cell walls. The peptidoglycan layer is important for cell wall structural integrity,[6] especially in gram-positive organisms, being the outermost and primary component of the wall. The final transpeptidation step in the synthesis of the peptidoglycan is facilitated by DD-transpeptidases, also known as penicillin binding proteins (PBPs). PBPs vary in their affinity for penicillin and other β-lactam antibiotics. The number of PBPs varies between bacterial species.[11]
β-Lactam antibiotics are analogues of d-alanyl-d-alanine—the terminal amino acid residues on the precursor NAM/NAG-peptide subunits of the nascent peptidoglycan layer. The structural similarity between β-lactam antibiotics and d-alanyl-d-alanine facilitates their binding to the active site of PBPs. The β-lactam nucleus of the molecule irreversibly binds to (acylates) the Ser403 residue of the PBP active site. This irreversible inhibition of the PBPs prevents the final crosslinking (transpeptidation) of the nascent peptidoglycan layer, disrupting cell wall synthesis.[13] β-Lactam antibiotics block not only the division of bacteria, including cyanobacteria, but also the division of cyanelles, the photosynthetic organelles of the glaucophytes, and the division of chloroplasts of bryophytes. In contrast, they have no effect on the plastids of the highly developed vascular plants. This is supporting the endosymbiotic theory and indicates an evolution of plastid division in land plants.[14]
Under normal circumstances, peptidoglycan precursors signal a reorganisation of the bacterial cell wall and, as a consequence, trigger the activation of autolytic cell wall hydrolases. Inhibition of cross-linkage by β-lactams causes a build-up of peptidoglycan precursors, which triggers the digestion of existing peptidoglycan by autolytic hydrolases without the production of new peptidoglycan. As a result, the bactericidal action of β-lactam antibiotics is further enhanced.[citation needed]
Guanine oxidation
editAnother possibility that has been proposed to account for much of the cytotoxicity of β-lactams focuses on the oxidation of the guanine nucleotide in the bacterial nucleotide pool.[15] The incorporation of oxidized guanine nucleotide into DNA could cause cytotoxicity. Bacterial cytotoxicity could arise from incomplete repair of closely spaced 8-oxo-2'-deoxyguanosine lesions in the DNA resulting in double-strand breaks.[15]
Potency
editTwo structural features of β-lactam antibiotics have been correlated with their antibiotic potency.[16] The first is known as "Woodward's parameter", h, and is the height (in angstroms) of the pyramid formed by the nitrogen atom of the β-lactam as the apex and the three adjacent carbon atoms as the base.[17] The second is called "Cohen's parameter", c, and is the distance between the carbon atom of the carboxylate and the oxygen atom of the β-lactam carbonyl.[18] This distance is thought to correspond to the distance between the carboxylate-binding site and the oxyanion hole of the PBP enzyme. The best antibiotics are those with higher h values (more reactive to hydrolysis) and lower c values (better binding to PBPs).[16]
Modes of resistance
editBy definition, all β-lactam antibiotics have a β-lactam ring in their structure. The effectiveness of these antibiotics relies on their ability to reach the PBP intact and their ability to bind to the PBP. Hence, there are two main modes of bacterial resistance to β-lactams: enzymatic hydrolysis of the β-lactam ring and possession of altered penicillin-binding proteins.[citation needed]
Enzymatic hydrolysis of the β-lactam ring
editIf the bacterium produces the enzyme β-lactamase or the enzyme penicillinase, the enzyme will hydrolyse the β-lactam ring of the antibiotic, rendering the antibiotic ineffective.[19] (An example of such an enzyme is New Delhi metallo-beta-lactamase 1, discovered in 2009.) The genes encoding these enzymes may be inherently present on the bacterial chromosome or may be acquired via plasmid transfer (plasmid-mediated resistance), and β-lactamase gene expression may be induced by exposure to β-lactams.[citation needed]
The production of a β-lactamase by a bacterium does not necessarily rule out all treatment options with β-lactam antibiotics. In some instances, β-lactam antibiotics may be co-administered with a β-lactamase inhibitor. For example, Augmentin (FGP) is made of amoxicillin (a β-lactam antibiotic) and clavulanic acid (a β-lactamase inhibitor). The clavulanic acid is designed to overwhelm all β-lactamase enzymes, and effectively serve as an antagonist so that the amoxicillin is not affected by the β-lactamase enzymes. Another β-lactam/β-lactamase inhibitor combination is piperacillin/tazobactam with a broad spectrum of antibacterial activity that includes gram-positive and -negative aerobic and anaerobic bacteria. The addition of tazobactam to piperacillin has enhanced its stability against a wide range of β-lactamase enzymes including some Extended-Spectrum β-lactamases.[20]
Other β-lactamase inhibitors such as boronic acids are being studied in which they irreversibly bind to the active site of β-lactamases. This is a benefit over clavulanic acid and similar β-lactam competitors, because they cannot be hydrolysed, and therefore rendered useless. Extensive research is currently being done to develop tailored boronic acids to target different isozymes of beta-lactamases.[21]
However, in all cases where infection with β-lactamase-producing bacteria is suspected, the choice of a suitable β-lactam antibiotic should be carefully considered prior to treatment. In particular, choosing appropriate β-lactam antibiotic therapy is of utmost importance against organisms which harbor some level of β-lactamase expression. In this case, failure to use the most appropriate β-lactam antibiotic therapy at the onset of treatment could result in selection for bacteria with higher levels of β-lactamase expression, thereby making further efforts with other β-lactam antibiotics more difficult.[22]
In the context of medical pharmacology, penicillins, cephalosporins, and carbapenems, while all have the β-lactam ring that serves as the fundamental structure, also have an auxiliary ring that carries a carboxylate group that is positioned on the same side as the carbonyl group within the β-lactam ring, and, as such, this structural configuration is critical to their antimicrobial activity.[23] Bacterial resistance to these antibiotics primarily occurs through the production of β-lactamases, enzymes that hydrolyze the amide bond of the β-lactam ring, thereby eliminating the antimicrobial activity of these antibiotics. This resistance mechanism underscores the importance of the structural integrity of the β-lactam ring for the antibiotic's function.[24] The color change from colorless or light yellow to amber or even red in an aqueous solution of a β-lactam antibiotic can denote β-lactamase hydrolysis of amide bonds in the β-lactam ring.[25][26] This is often observed with a chromogenic β-lactamase substrate like ceftriaxone, merapenem, or nitrocefin,[27] that undergoes a distinctive color change from yellow to red as the amide bond in the β-lactam ring is hydrolyzed by β-lactamase.[27][28] This color change is a visual indicator of the presence and activity of β-lactamase enzymes, which are responsible for conferring resistance to β-lactam antibiotics in many bacterial species. The hydrolysis of the β-lactam ring by β-lactamase enzymes renders the antibiotic ineffective, thereby allowing the bacteria to survive in the presence of the antibiotic.[28] Some β-lactam antibiotics like ceftriaxone and meropenem are known to be relatively unstable in solution, especially when stored for extended periods, and degrade in an aqueous solution even without the presence of β-lactamase.[29] For ceftriaxone, the color of solutions can range from light yellow to amber, depending on the length of storage, concentration, and diluent used.[30][31] A study found that meropenem concentrations dropped to 90% of the initial concentration at 7.4 hours at 22°C and 5.7 hours at 33°C, indicating degradation over time.[29]
Possession of altered penicillin-binding proteins
editAs a response to the use of β-lactams to control bacterial infections, some bacteria have evolved penicillin binding proteins with novel structures. β-Lactam antibiotics cannot bind as effectively to these altered PBPs, and, as a result, the β-lactams are less effective at disrupting cell wall synthesis. Notable examples of this mode of resistance include methicillin-resistant Staphylococcus aureus (MRSA)[32] and penicillin-resistant Streptococcus pneumoniae. Altered PBPs do not necessarily rule out all treatment options with β-lactam antibiotics.[medical citation needed]
Nomenclature
editβ-Lactams are classified according to their core ring structures.[33]
- β-Lactams fused to saturated five-membered rings:
- β-Lactams containing thiazolidine rings are named penams.
- β-Lactams containing pyrrolidine rings are named carbapenams.
- β-Lactams fused to oxazolidine rings are named oxapenams or clavams.
- β-Lactams fused to unsaturated five-membered rings:
- β-Lactams containing 2,3-dihydrothiazole rings are named penems.
- β-Lactams containing 2,3-dihydro-1H-pyrrole rings are named carbapenems.
- β-Lactams fused to unsaturated six-membered rings:
- β-Lactams containing 3,6-dihydro-2H-1,3-thiazine rings are named cephems.
- β-Lactams containing 1,2,3,4-tetrahydropyridine rings are named carbacephems.
- β-Lactams containing 3,6-dihydro-2H-1,3-oxazine rings are named oxacephems.
- β-Lactams not fused to any other ring are named monobactams.
By convention, the bicyclic β-lactams are numbered starting with the position occupied by sulfur in the penams and cephems, regardless of which atom it is in a given class. That is, position 1 is always adjacent to the β-carbon of β-lactam ring. The numbering continues clockwise from position one until the β-carbon of β-lactam is reached, at which point numbering continues counterclockwise around the lactam ring to number the remaining to carbons. For example, the nitrogen atom of all bicyclic β-lactams fused to five-membered rings is labelled position 4, as it is in penams, while in cephems, the nitrogen is position 5.[medical citation needed]
The numbering of monobactams follows that of the IUPAC; the nitrogen atom is position 1, the carbonyl carbon is 2, the α-carbon is 3, and the β-carbon 4.[medical citation needed]
Biosynthesis
editTo date, two distinct methods of biosynthesizing the β-lactam core of this family of antibiotics have been discovered. The first pathway discovered was that of the penams and cephems. This path begins with a nonribosomal peptide synthetase (NRPS), ACV synthetase (ACVS), which generates the linear tripeptide δ-(L-α-aminoadipyl)-L-cysteine-D-valine (ACV). ACV is oxidatively cyclized (two cyclizations by a single enzyme) to bicyclic intermediate isopenicillin N by isopenicillin N synthase (IPNS) to form the penam core structure.[34] Various transamidations lead to the different natural penicillins.
The biosynthesis of cephems branch off at isopenicillin N by an oxidative ring expansion to the cephem core. As with the penams, the variety of cephalosporins and cephamycins come from different transamidations, as is the case for the penicillins.[citation needed]
While the ring closure in penams and cephems is between positions 1 and 4 of the β-lactam and is oxidative, the clavams and carbapenems have their rings closed between positions 1 and 2 of the ring. β-lactam synthetases are responsible for these cyclizations, and the carboxylate of the open-ring substrates is activated by ATP.[35] In clavams, the β-lactam is formed prior to the second ring; in carbapenems, the β-lactam ring is closed second in sequence.[citation needed]
The biosynthesis of the β-lactam ring of tabtoxin mirrors that of the clavams and carbapenems. The closure of the lactam ring in the other monobactams, such as sulfazecin and the nocardicins, may involve a third mechanism involving inversion of configuration at the β-carbon.[36]
See also
editReferences
edit- ^ Holten KB, Onusko EM (August 2000). "Appropriate prescribing of oral beta-lactam antibiotics". American Family Physician. 62 (3): 611–20. PMID 10950216. Archived from the original on June 6, 2011. Retrieved November 8, 2008.
- ^ Yao, JDC, Moellering, RC Jr. (2007). "Antibacterial agents". In Murray, PR, et al. (eds.). Manual of Clinical Microbiology (9th ed.). Washington D.C.: ASM Press. Cited in Non-Penicillin Beta Lactam Drugs: A CGMP Framework for Preventing Cross-Contamination (Report). U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER). April 2013. Archived from the original on December 13, 2019. Retrieved May 27, 2019 – via US FDA website.
- ^ Elander RP (2003). "Industrial production of β-lactam antibiotics". Applied Microbiology and Biotechnology. 61 (5–6): 385–392. doi:10.1007/s00253-003-1274-y. PMID 12679848. S2CID 43996071.
- ^ Houbraken J, Frisvad JC, Samson RA (2011). "Fleming's penicillin producing strain is not Penicillium chrysogenum but P. rubens". IMA Fungus. 2 (1): 87–95. doi:10.5598/imafungus.2011.02.01.12. PMC 3317369. PMID 22679592.
- ^ Pathak A, Nowell RW, Wilson CG, Ryan MJ, Barraclough TG (September 2020). "Comparative genomics of Alexander Fleming's original Penicillium isolate (IMI 15378) reveals sequence divergence of penicillin synthesis genes". Scientific Reports. 10 (1): Article 15705. Bibcode:2020NatSR..1015705P. doi:10.1038/s41598-020-72584-5. PMC 7515868. PMID 32973216.
- ^ a b Pandey N, Cascella M (2020). "Beta lactam antibiotics". StatPearls. PMID 31424895. Archived from the original on December 15, 2020. Retrieved May 5, 2020.
- ^ Nau R, Sörgel F, Eiffert H (October 2010). "Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections". Clinical Microbiology Reviews. 23 (4): 858–83. doi:10.1128/CMR.00007-10. PMC 2952976. PMID 20930076.
- ^ a b c Rossi S (ed.) (2004). Australian Medicines Handbook 2004. Adelaide: Australian Medicines Handbook. ISBN 0-9578521-4-2.
- ^ Pichichero ME (April 2005). "A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients". Pediatrics. 115 (4): 1048–57. doi:10.1542/peds.2004-1276. PMID 15805383. S2CID 21246804.
- ^ Liccardi G, Caminati M, Senna G, Calzetta L, Rogliani P (October 2017). "Anaphylaxis and intimate behaviour". Current Opinion in Allergy and Clinical Immunology. 17 (5): 350–355. doi:10.1097/ACI.0000000000000386. ISSN 1473-6322. PMID 28742538. S2CID 13925217. Archived from the original on April 25, 2023. Retrieved February 27, 2021.
- ^ a b Miyachiro MM, Contreras-Martel C, Dessen A (2019). "Penicillin-Binding Proteins (PBPS) and Bacterial Cell Wall Elongation Complexes". Macromolecular Protein Complexes II: Structure and Function. Subcellular Biochemistry. Vol. 93. pp. 273–289. doi:10.1007/978-3-030-28151-9_8. ISBN 978-3-030-28150-2. PMID 31939154. S2CID 210814189.
- ^ Cushnie TP, O'Driscoll NH, Lamb AJ (2016). "Morphological and ultrastructural changes in bacterial cells as an indicator of antibacterial mechanism of action". Cellular and Molecular Life Sciences. 73 (23): 4471–4492. doi:10.1007/s00018-016-2302-2. hdl:10059/2129. PMC 11108400. PMID 27392605. S2CID 2065821. Archived from the original on October 7, 2017. Retrieved May 5, 2020.
- ^ Fisher JF, Meroueh SO, Mobashery S (2005). "Bacterial resistance to β-lactam antibiotics: compelling opportunism, compelling opportunity". Chemical Reviews. 105 (2): 395–424. doi:10.1021/cr030102i. PMID 15700950.
- ^ Kasten B, Reski R (January 1, 1997). "β-Lactam antibiotics inhibit chloroplast division in a moss (Physcomitrella patens) but not in tomato (Lycopersicon esculentum)". Journal of Plant Physiology. 150 (1): 137–140. Bibcode:1997JPPhy.150..137K. doi:10.1016/S0176-1617(97)80193-9.
- ^ a b Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC (April 20, 2012). "Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics". Science. 336 (6079): 315–319. Bibcode:2012Sci...336..315F. doi:10.1126/science.1219192. PMC 3357493. PMID 22517853.
- ^ a b Nangia A, Biradha K, Desiraju GR (1996). "Correlation of biological activity in β-lactam antibiotics with Woodward and Cohen structural parameters—a Cambridge database study". Journal of the Chemical Society, Perkin Transactions 2 (5): 943–953. doi:10.1039/p29960000943. ISSN 1364-5471.
- ^ Woodward RB (May 16, 1980). "Penems and related substances". Philosophical Transactions of the Royal Society of London B: Biological Sciences. 289 (1036): 239–250. Bibcode:1980RSPTB.289..239W. doi:10.1098/rstb.1980.0042. ISSN 0962-8436. PMID 6109320.
- ^ Cohen NC (February 1, 1983). ".beta.-Lactam antibiotics: geometrical requirements for antibacterial activities". Journal of Medicinal Chemistry. 26 (2): 259–264. doi:10.1021/jm00356a027. ISSN 0022-2623. PMID 6827544.
- ^ Drawz SM, Bonomo RA (2010). "Three decades of β-lactamase inhibitors". Clinical Microbiology Reviews. 23 (1): 160–201. doi:10.1128/CMR.00037-09. PMC 2806661. PMID 20065329.
- ^ Gin A, Dilay L, Karlowsky JA, Walkty A, Rubinstein E, Zhanel GG (June 2007). "Piperacillin–tazobactam: a β-lactam/β-lactamase inhibitor combination". Expert Review of Anti-infective Therapy. 5 (3): 365–383. doi:10.1586/14787210.5.3.365. ISSN 1478-7210. PMID 17547502. S2CID 68837323.
- ^ Leonard DA, Bonomo RA, Powers RA (November 19, 2013). "Class D β-Lactamases: a reappraisal after five decades". Accounts of Chemical Research. 46 (11): 2407–2415. doi:10.1021/ar300327a. ISSN 0001-4842. PMC 4018812. PMID 23902256.
- ^ Macdougall C (2011). "Beyond susceptible and resistant Part I: treatment of infections due to Gram-negative organisms with inducible B-lactamases". Journal of Pediatric Pharmacology and Therapeutics. 16 (1): 23–30. doi:10.5863/1551-6776-16.1.23. PMC 3136230. PMID 22477821.
- ^ Kim D, Kim S, Kwon Y, Kim Y, Park H, Kwak K, Lee H, Lee JH, Jang KM, Kim D, Lee SH, Kang LW (March 2023). "Structural Insights for β-Lactam Antibiotics". Biomol Ther (Seoul). 31 (2): 141–147. doi:10.4062/biomolther.2023.008. PMC 9970833. PMID 36788654.
- ^ Ahmadvand P, Avillan JJ, Lewis JA, Call DR, Kang C (May 2022). "Characterization of Interactions between CTX-M-15 and Clavulanic Acid, Desfuroylceftiofur, Ceftiofur, Ampicillin, and Nitrocefin". Int J Mol Sci. 23 (9): 5229. doi:10.3390/ijms23095229. PMC 9100253. PMID 35563620.
- ^ "Archived copy" (PDF). Archived (PDF) from the original on February 16, 2022. Retrieved February 22, 2024.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Christenson JC, Korgenski EK (2008). "Laboratory Diagnosis of Infection Due to Bacteria, Fungi, Parasites, and Rickettsiae". Principles and Practice of Pediatric Infectious Disease. W.B. Saunders. pp. 1341–1352. doi:10.1016/B978-0-7020-3468-8.50292-3. ISBN 978-0-7020-3468-8.
- ^ a b "Archived copy". Archived from the original on February 22, 2024. Retrieved February 22, 2024.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ a b Palzkill T (January 2013). "Metallo-β-lactamase structure and function". Ann N Y Acad Sci. 1277 (1): 91–104. Bibcode:2013NYASA1277...91P. doi:10.1111/j.1749-6632.2012.06796.x. PMC 3970115. PMID 23163348.
- ^ a b Fawaz S, Barton S, Whitney L, Swinden J, Nabhani-Gebara S (2019). "Stability of Meropenem After Reconstitution for Administration by Prolonged Infusion". Hospital Pharmacy. 54 (3): 190–196. doi:10.1177/0018578718779009. PMC 6535930. PMID 31205331. Archived from the original on February 27, 2024. Retrieved February 22, 2024.
- ^ "Ceftriaxone: Package Insert". Archived from the original on April 2, 2023.
- ^ https://web.archive.org/web/20240222172948/https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/0550585s063lbl.pdf [bare URL PDF]
- ^ Ubukata K, Nonoguchi R, Matsuhashi M, Konno M (1989). "Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein". Journal of Bacteriology. 171 (5): 2882–5. doi:10.1128/jb.171.5.2882-2885.1989. PMC 209980. PMID 2708325.
- ^ Dalhoff A, Janjic N, Echols R (2006). "Redefining penems". Biochemical Pharmacology. 71 (7): 1085–1095. doi:10.1016/j.bcp.2005.12.003. PMID 16413506.
- ^ Lundberg M, Siegbahn PE, Morokuma K (2008). "The mechanism for isopenicillin N synthase from density-functional modeling highlights the similarities with other enzymes in the 2-His-1-carboxylate family". Biochemistry. 47 (3): 1031–1042. doi:10.1021/bi701577q. PMID 18163649. Archived from the original on September 22, 2017. Retrieved October 28, 2017.
- ^ Bachmann BO, Li R, Townsend CA (1998). "β-lactam synthetase: a new biosynthetic enzyme". Proceedings of the National Academy of Sciences of the United States of America. 95 (16): 9082–9086. Bibcode:1998PNAS...95.9082B. doi:10.1073/pnas.95.16.9082. PMC 21295. PMID 9689037.
- ^ Townsend CA, Brown AM, Nguyen LT (1983). "Nocardicin A: stereochemical and biomimetic studies of monocyclic β-lactam formation". Journal of the American Chemical Society. 105 (4): 919–927. doi:10.1021/ja00342a047.