Uranium hexachloride (UCl6) is an inorganic chemical compound of uranium in the +6 oxidation state.[1][2] UCl6 is a metal halide composed of uranium and chlorine. It is a multi-luminescent dark green crystalline solid with a vapor pressure between 1-3 mmHg at 373.15 K.[3] UCl6 is stable in a vacuum, dry air, nitrogen and helium at room temperature. It is soluble in carbon tetrachloride (CCl4). Compared to the other uranium halides, little is known about UCl6.
| |
| Names | |
|---|---|
| IUPAC name
Uranium(VI) chloride
| |
| Other names
Uranium hexachloride
Peruranic chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| UCl6 | |
| Molar mass | 450.745 g/mol |
| Appearance | dark green crystalline solid |
| Density | 3.6 g/cm3 |
| Melting point | 177 °C (351 °F; 450 K) |
| Related compounds | |
Other anions
|
Uranium hexafluoride |
Other cations
|
Tungsten hexachloride |
Related uranium chlorides
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
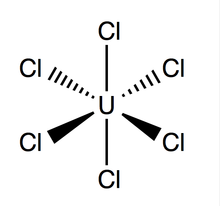
Structure and Bonding
editUranium hexachloride has an octahedral geometry, with point group Oh. Its lattice (dimensions: 10.95 ± 0.02 Å x 6.03 ± 0.01 Å) is hexagonal in shape with three molecules per cell; the average theoretical U-Cl bond is 2.472 Å long (the experimental U-Cl length found by X-ray diffraction is 2.42 Å),[4] and the distance between two adjacent chlorine atoms is 3.65 Å.
Chemical properties
editUranium hexachloride is a highly hygroscopic compound and decomposes readily when exposed to ordinary atmospheric conditions.[5] therefore it should be handled in either a vacuum apparatus or in a dry box.
Thermal decomposition
editUCl6 is stable up to temperatures between 120 °C and 150 °C. The decomposition of UCl6 results in a solid phase transition from one crystal form of UCl6 to another more stable form.[6] However, the decomposition of gaseous UCl6 produces UCl5. The activation energy for this reaction is about 40 kcal per mole.
- 2 UCl6 (g) → 2 UCl5 (s) + Cl2 (g)
Solubility
editUCl6 is not a very soluble compound. It dissolves in CCl4 to give a brown solution. It is slightly soluble in isobutyl bromide and in fluorocarbon (C7F16).[6]
| Solvents | Temperature (°C) | Grams of UCl6/100g of solution |
|---|---|---|
| CCl4 | −18 | 2.64 |
| CCl4 | 0 | 4.9 |
| CCl4 | 20 | 7.8 |
| 6.6% Cl2 : 93.4% CCl4 | −20 | 2.4 |
| 12.5% Cl2 : 87.5% CCl4 | −20 | 2.23 |
| 12.5% Cl2 : 87.5% CCl4 | 0 | 3.98 |
| Liquid Cl2 | −33 | 2.20 |
| CH3Cl | −24 | 1.16 |
| Benzene | 80 | Insoluble |
| Freon 113 | 45 | 1.83 |
Reaction with hydrogen fluoride
editWhen UCl6 is reacted with purified anhydrous liquid hydrogen fluoride (HF) at room temperature produces UF5.[6]
- 2 UCl6 + 10 HF → 2 UF5 + 10 HCl + Cl2
Synthesis
editUranium hexachloride can be synthesized from the reaction of uranium trioxide (UO3) with a mixture of liquid CCl4 and hot chlorine (Cl2). The yield can be increased if the reaction carried out in the presence of UCl5.[7] The UO3 is converted to UCl5, which in turn reacts with the excess Cl2 to form UCl6. It requires a substantial amount of heat for the reaction to take place; the temperature range is from 65 °C to 170 °C depending on the amount of reactant (ideal temperature 100 °C - 125 °C). The reaction is carried out in a closed gas-tight vessel (for example a glovebox) that can withstand the pressure that builds up.
Step 1: 2 UO3 + 5 Cl2 → 2 UCl5 + 3 O2
Step 2: 2 UCl5 + Cl2 → 2 UCl6
Overall reaction: 2 UO3 + 6 Cl2 → 2 UCl6 + 3 O2
This metal hexahalide can also be synthesized by blowing Cl2 gas over sublimed UCl4 at 350 °C.[8]
Step 1: 2 UCl4 + Cl2 → 2 UCl5
Step 2: 2 UCl5 + Cl2 → 2 UCl6
Overall Reaction: UCl4 + Cl2 → UCl6
References
edit- ^ Zachariasen, W. H. (1948). "Crystal chemical studies of the 5f-series of elements. V. The crystal structure of uranium hexachloride". Acta Crystallographica. 1 (6): 285–287. Bibcode:1948AcCry...1..285Z. doi:10.1107/S0365110X48000788.
- ^ Taylor, J. C.; Wilson, P. W. (1974). "Neutron and X-ray powder diffraction studies of the structure of uranium hexachloride". Acta Crystallographica Section B. 30 (6): 1481. Bibcode:1974AcCrB..30.1481T. doi:10.1107/S0567740874005115.
- ^ Van Dyke, R. E.; Evers, E. C. (1955). "Preparation of Uranium Hexachloride". Google Patents: 2.
- ^ Batista, E. R.; Martin, R. L.; Hay, P. J. (2004). "Density Functional Investigations of the Properties and Thermodynamics of UFn and UCln (n=1,...,6)". J. Chem. Phys. 121 (22): 11104–11. doi:10.1063/1.1811607. PMID 15634063.
- ^ Lipkin, D.; Wessman, S. (1955). "Process and Apparatus for protecting Uranium hexachloride from Deterioration and Contamination". Google Patents: 2.
- ^ a b c Katz, J.J.; Rabinowitch, E. (1951). The Chemistry of Uranium. Ann Arbor: The McGraw-Hill Book Company.
- ^ Van Dyke, R. E.; Evers, E. C. (1955). "Preparation of Uranium Hexachloride". Google Patents: 2.
- ^ Thornton, G.; Edelstein, N.; Rösch, N.; Woodwark, D.R.; Edgell, R.G. (1979). "The Electronic Structure of UCl6: Photoelectron Spectra and Scattered Wave Xα Calculations". J. Chem. Phys. 70 (11): 6. Bibcode:1979JChPh..70.5218T. doi:10.1063/1.437313.