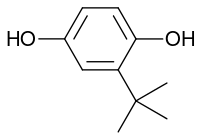
tert-Butylhydroquinone (TBHQ, tertiary butylhydroquinone) is a synthetic aromatic organic compound which is a type of phenol. It is a derivative of hydroquinone, substituted with a tert-butyl group.
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-tert-Butylbenzene-1,4-diol | |
| Other names
TBHQ(i)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.016.139 |
| EC Number |
|
| E number | E319 (antioxidants, ...) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H14O2 | |
| Molar mass | 166.220 g·mol−1 |
| Appearance | Tan powder |
| Density | 1.050 g/mL |
| Melting point | 127 to 129 °C (261 to 264 °F; 400 to 402 K) |
| Boiling point | 273 °C (523 °F; 546 K) |
| Slightly soluble | |
| Acidity (pKa) | 10.80±0.18 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Harmful |
| GHS labelling: | |
 
| |
| Warning | |
| H302, H312, H315, H317, H319, H335, H400 | |
| P261, P264, P270, P271, P272, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P333+P313, P337+P313, P362, P363, P391, P403+P233, P405, P501 | |
| Flash point | 171 °C (340 °F; 444 K) |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related compounds
|
Butylated hydroxyanisole (BHA) 4-tert-Butylcatechol (TBC) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Applications
editFood preservative
editIn foods, TBHQ is used as a preservative for unsaturated vegetable oils and many edible animal fats. It does not cause discoloration even in the presence of iron, and does not change flavor or odor of the material to which it is added.[1] It can be combined with other preservatives such as butylated hydroxyanisole (BHA). As a food additive, its E number is E319. It is added to a wide range of foods. Its primary advantage is extending storage life.[1]
Other
editIn perfumery, it is used as a fixative to lower the evaporation rate and improve stability.
It is used industrially as a stabilizer to inhibit autopolymerization of organic peroxides.
It is used as an antioxidant in biodiesel.[2]
It is also added to varnishes, lacquers, resins, and oil-field additives.
Safety and regulation
editThe European Food Safety Authority (EFSA) and the United States Food and Drug Administration (FDA)[3] have evaluated TBHQ and determined that it is safe to consume at the concentration allowed in foods.[4] The FDA[5] and European Union[4] both set an upper limit of 0.02% (200mg/kg) of the oil or fat content in foods. At very high doses, it has some negative health effects on lab animals, such as producing precursors to stomach tumors and damage to DNA.[6]
A number of studies have shown that prolonged exposure to very high doses of TBHQ may be carcinogenic,[7] especially for stomach tumors.[8] Other studies, however, have shown opposite effects including inhibition against HCA-induced carcinogenesis (by depression of metabolic activation) for TBHQ and other phenolic antioxidants (TBHQ was one of several, and not the most potent).[9] The EFSA considers TBHQ to be noncarcinogenic.[4] A 1986 review of scientific literature concerning the toxicity of TBHQ determined that a wide margin of safety exists between the levels of intake by humans and the doses that produce adverse effects in animal studies.[10]
In addition, TBHQ has been identified by high-throughput screening as having potential immunotoxic effects.[11]
There have been reports of vision disturbances in individuals exposed to this chemical.[12]
References
edit- ^ a b Fats and oils: formulating and processing for applications, Richard D. O'Brien, page 168
- ^ Almeida, Eduardo S.; Portela, Flaysner M.; Sousa, Raquel M.F.; Daniel, Daniela; Terrones, Manuel G.H.; Richter, Eduardo M.; Muñoz, Rodrigo A.A. (2011). "Behaviour of the antioxidant tert-butylhydroquinone on the storage stability and corrosive character of biodiesel". Fuel. 90 (11): 3480–3484. Bibcode:2011Fuel...90.3480A. doi:10.1016/j.fuel.2011.06.056.
- ^ "Food Additive Status List". U.S. Food and Drug Administration. Retrieved June 11, 2016.
- ^ a b c "Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to tertiary-Butylhydroquinone (TBHQ)". European Food Safety Authority, 12 July 2004
- ^ 21 C.F.R. § 172.185
- ^ Tert-Butylhydroquinone - safety summary from The International Programme on Chemical Safety
- ^ Gharavi N, El-Kadi A (2005). "tert-Butylhydroquinone is a novel aryl hydrocarbon receptor ligand". Drug Metab Dispos. 33 (3): 365–72. doi:10.1124/dmd.104.002253. PMID 15608132. S2CID 5963778.
- ^ Hirose, Masao; Yada, Hideaki; Hakoi, Kazuo; Takahashi, Satoru; Ito, Nobuyuki; et al. (1993). "Modification of carcinogenesis by -tocopherol, t-butylhydro-quinone, propyl gallate and butylated hydroxytoluene in a rat multi-organ carcinogenesis model". Carcinogenesis. 14 (1). Oxford University Press: 2359–2364. doi:10.1093/carcin/14.11.2359. PMID 8242867.
- ^ Hirose M, Takahashi S, Ogawa K, Futakuchi M, Shirai T, Shibutani M, Uneyama C, Toyoda K, Iwata H (1999). "Chemoprevention of heterocyclic amine-induced carcinogenesis by phenolic compounds in rats". Cancer Lett. 143 (2): 173–8. doi:10.1016/S0304-3835(99)00120-2. PMID 10503899.
- ^ Vanesch, G (1986). "Toxicology of tert-butylhydroquinone (TBHQ)". Food and Chemical Toxicology. 24 (10–11): 1063–5. doi:10.1016/0278-6915(86)90289-9. PMID 3542758.
- ^ Naidenko, Olga V.; Andrews, David Q.; Temkin, Alexis M.; Stoiber, Tasha; Uche, Uloma Igara; Evans, Sydney; Perrone-Gray, Sean (2021-03-24). "Investigating Molecular Mechanisms of Immunotoxicity and the Utility of ToxCast for Immunotoxicity Screening of Chemicals Added to Food". International Journal of Environmental Research and Public Health. 18 (7): 3332. doi:10.3390/ijerph18073332. ISSN 1660-4601. PMC 8036665. PMID 33804855.
- ^
- "T-BUTYLHYDROQUINONE - National Library of Medicine HSDB Database". toxnet.nlm.nih.gov. Retrieved 2019-11-21.
- O'Donoghue, J.L. (ed.). Neurotoxicity of Industrial and Commercial Chemicals. Volume I. Boca Raton, FL: CRC Press, Inc., 1985., p. 129 – HSDB cites this source.