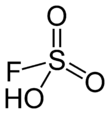
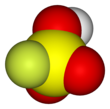
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula HSO3F. It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, H2SO4, substituting a fluorine atom for one of the hydroxyl groups. It is a colourless liquid, although commercial samples are often yellow.[2]
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sulfurofluoridic acid
| |||
| Systematic IUPAC name
Fluorosulfuric acid[citation needed] | |||
| Other names
Fluorosulfonic acid,
Fluorosulphonic acid, Fluorinesulfonic acid, Fluorinesulphonic acid, Fluoridosulfonic acid, Fluoridosulphonic acid, Sulfuric fluorohydrin, Epoxysulfonyl fluoride | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.029.227 | ||
| EC Number |
| ||
| MeSH | Fluorosulfonic+acid | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1777 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| FHO3S | |||
| Molar mass | 100.06 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.726 g cm−3 | ||
| Melting point | −87.5 °C; −125.4 °F; 185.7 K | ||
| Boiling point | 165.4 °C; 329.6 °F; 438.5 K | ||
| Acidity (pKa) | -10 | ||
| Conjugate base | Fluorosulfate | ||
| Structure | |||
| Tetragonal at S | |||
| Tetrahedral at S | |||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H314, H332[1] | |||
| P261, P271, P280, P303+P361+P353, P304+P340+P310, P305+P351+P338[1] | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | ICSC 0996 | ||
| Related compounds | |||
Related compounds
|
Antimony pentafluoride Trifluoromethanesulfonic acid Hydrofluoric acid Sulfurous acid Sulfuric acid Sulfur hexafluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Properties
editFluorosulfuric acid is a free-flowing colorless liquid. It is soluble in polar organic solvents (e.g. nitrobenzene, acetic acid, and ethyl acetate), but poorly soluble in nonpolar solvents such as alkanes.
HSO3F is one of the strongest known simple Brønsted acids.[3] It has an H0 value of −15.1 compared to −12 for sulfuric acid. The combination of HSO3F and the Lewis acid antimony pentafluoride produces "Magic acid", which is a far stronger protonating agent. These acids are categorized as "superacids", acids stronger than 100% sulfuric acid.
Reflecting its strong acidity, HSO3F dissolves almost all organic compounds that are even weak proton acceptors.[4] HSO3F hydrolyzes slowly to hydrogen fluoride (HF) and sulfuric acid. The related triflic acid (CF3SO3H) retains the high acidity of HSO3F but is more hydrolytically stable. The self-ionization of fluorosulfonic acid also occurs:
- 2 HSO3F ⇌ [H2SO3F]+ + [SO3F]− K = 4.0 × 10−8 (at 298 K)
HSO3F isomerizes alkanes and catalyzes the alkylation of hydrocarbons with alkenes,[5] although it is unclear if such applications are of commercial importance. It can also be used as a laboratory fluorinating agent.[6]
Production
editFluorosulfuric acid is prepared by the reaction of HF and sulfur trioxide:[2]
- SO3 + HF → HSO3F
Alternatively, KHF2 or CaF2 can be treated with oleum at 250 °C. Once freed from HF by sweeping with an inert gas, HSO3F can be distilled in a glass apparatus.[6]
Safety
editFluorosulfuric acid is considered to be highly toxic and extremely corrosive. It hydrolyzes to release HF. Addition of water to HSO3F is similar to, and even more violent than, the addition of water to sulfuric acid.
See also
edit- Chlorosulfuric acid
- Sulfuryl fluoride
- Methyl fluorosulfonate, an organic ester of HSO3F
- Trifluoromethylsulfonic acid
References
edit- ^ a b Sigma-Aldrich Co., Fluorosulfonic acid. Retrieved on 2024-01-27.
- ^ a b Erhardt Tabel, Eberhard Zirngiebl, Joachim Maas "Fluorosulfuric Acid" in "Ullmann's Encyclopedia of Industrial Chemistry" 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a11_431
- ^ Christopher A. Reed "Myths about the Proton. The Nature of H+ in Condensed Media" Acc. Chem. Res., 2013, 46 (11), pp 2567–2575. doi:10.1021/ar400064q
- ^ Olah, G. A.; Prakash, G. K.; Wang, Q.; Li, X.-Y. (2001). "Fluorosulfuric Acid". Encyclopedia of Reagents for Organic Synthesis. Encyclopedia of Reagents for Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rf014. ISBN 0471936235.
- ^ Olah, G.; Farooq, O.; Husain, A.; Ding, N.; Trivedi, N.; Olah, J. (1991). "Superacid HSO3F/HF-Catalyzed Butane Isomerisation". Catalysis Letters. 10 (3–4): 239–247. doi:10.1007/BF00772077. S2CID 94408218.
- ^ a b Cotton, F. A.; Wilkinson, G. (1980). Advanced Inorganic Chemistry (4th ed.). New York: Wiley. p. 246. ISBN 0-471-02775-8.