The rhinovirus (from the Ancient Greek: ῥίς, romanized: rhis "nose", gen ῥινός, romanized: rhinos "of the nose", and the Latin: vīrus) is a positive-sense, single-stranded RNA virus belonging to the genus Enterovirus in the family Picornaviridae. Rhinovirus is the most common viral infectious agent in humans and is the predominant cause of the common cold.[1]
| Rhinovirus | |
|---|---|
| |
| Rhinovirus | |
| Scientific classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Pisuviricota |
| Class: | Pisoniviricetes |
| Order: | Picornavirales |
| Family: | Picornaviridae |
| Genus: | Enterovirus |
| Groups included | |
| |
| Cladistically included but traditionally excluded taxa | |
| |
The three species of rhinovirus (A, B, and C) include at least 165 recognized types that differ according to their surface antigens or genetics.[2] They are among the smallest viruses, with diameters of about 30 nanometers. By comparison, other viruses, such as smallpox and vaccinia, are around ten times larger at about 300 nanometers, while influenza viruses are around 80–120 nm.
Rhinoviruses are transmitted through aerosols, respiratory droplets, fomites, and direct person-to-person contact.[3] They primarily infect nasal epithelial cells in the airway and cause mild symptoms such as sore throat, cough, and nasal congestion.[4][5] However, rhinovirus infection can cause more severe disease in infants,[6][7] the elderly, and the immunocompromised. Rhinoviruses are also recognized as a major cause of asthma exacerbations.[8]
As of April 2024, there are no FDA-approved vaccines or antiviral treatments for rhinovirus infection.[5]
History
editIn 1953, when a cluster of nurses developed a mild respiratory illness, Winston Price, from the Johns Hopkins University, took nasal passage samples and isolated the first rhinovirus, which he called the JH virus, named after Johns Hopkins.[9][10] His findings were published in 1956.[11]
In 2006, advancements in molecular testing techniques for identifying rhinoviruses in clinical specimens led to the discovery of rhinovirus C species in samples from Queensland, Australia and New York City, United States. The ICTV formally designated RV-C as a separate species in 2009.[4]
Transmission
editRhinoviruses may be spread via airborne aerosols, respiratory droplets and from fomites (contaminated surfaces), including direct person-to-person contact.[3] Rhinoviruses can survive on surfaces such as stainless steel or plastic for several hours. Airborne precautions[12] are likely effective in reducing transmission, while other precautions such as hand-washing or cleaning surfaces with disinfectants are known effective in preventing rhinovirus transmission.[13]
Signs and symptoms
editRhinoviruses are the primary cause of the common cold. Symptoms include sore throat, runny nose, nasal congestion, sneezing and cough; sometimes accompanied by muscle aches, fatigue, malaise, headache, muscle weakness, or loss of appetite. Fever and extreme exhaustion are less common in rhinovirus infection compared to influenza.
Epidemiology
editRhinoviruses can be detected year-round; however, the incidence of rhinovirus is higher in the autumn and winter, with most infections occurring between September and April in the northern hemisphere.[14] The seasonality may be due to the start of the school year and to people spending more time indoors thereby increasing the chance of transmission of the virus.[15] Lower ambient temperatures, especially outdoors, may also be a factor given that rhinoviruses preferentially replicate at 33 °C (91.4 °F) as opposed to 37 °C (98.6 °F).[14][16] Other climate factors such as humidity may influence rhinovirus seasonality.[14] Young children (<5 years old) experience a high rate of infection which can be detected in community surveillance studies of children up to 34% of the year.[17]
Those most affected by rhinoviruses are infants, the elderly, and immunocompromised people.[4]
Pathogenesis
editThe primary route of entry for human rhinoviruses is the upper respiratory tract (mouth and nose). Rhinovirus A and B use "major" ICAM-1 (Inter-Cellular Adhesion Molecule 1), also known as CD54 (Cluster of Differentiation 54), on respiratory epithelial cells, as receptors to bind to. Some subgroups under A and B uses the "minor" LDL receptor instead.[18] Rhinovirus C uses cadherin-related family member 3 (CDHR3) to mediate cellular entry.[19] As the virus replicates and spreads, infected cells release distress signals known as chemokines and cytokines (which in turn activate inflammatory mediators).
Infection occurs rapidly, with the virus adhering to surface receptors within 15 minutes of entering the respiratory tract. Just over 50% of individuals will experience symptoms within 2 days of infection. Only about 5% of cases will have an incubation period of less than 20 hours, and, at the other extreme, it is expected that 5% of cases would have an incubation period of greater than four and a half days.[20]
Human rhinoviruses preferentially grow at 33 °C (91.4 °F), notably colder than the average human body temperature of 37 °C (98.6 °F), hence the virus's tendency to infect the upper respiratory tract, where respiratory airflow is in continual contact with the (colder) extrasomatic environment.[14][16]
Rhinovirus A and C species viruses are more strongly associated with significant illness and wheezing, while rhinovirus B species are more commonly mild or asymptomatic.[4][21]
Taxonomy
editRhinovirus was formerly classified as a genus of the family Picornaviridae. The 39th Executive Committee (EC39) of the International Committee on Taxonomy of Viruses (ICTV) met in Canada during June 2007 with new taxonomic proposals. In April 2008, the International Committee on Taxonomy of Viruses voted and ratified the following changes:[23]
- 2005.264V.04 To remove the following species from the existing genus Rhinovirus in the family Picornaviridae:
- Human rhinovirus A
- Human rhinovirus B
- 2005.265V.04 To assign the following species to the genus Enterovirus in the family Picornaviridae:
- Human rhinovirus A
- Human rhinovirus B
- 2005.266V.04 To remove the existing genus Rhinovirus from the family Picornaviridae. Note: The genus Rhinovirus hereby disappears.
The merge is based on the grounds that the two "genera" of viruses are not significantly different in a virological sense. They have identical genome organizations and particle structures, and the phylogeny is not always monophyletic.[24]
In July 2009, the ICTV voted and ratified a proposal to add a third species, Human rhinovirus C to the genus Enterovirus.[25]
- 2008.084V.A.HRV-C-Sp 2008.084V To create a new species named Human rhinovirus C in the genus Enterovirus, family Picornaviridae.
There have been a total of 215 taxonomic proposals, which have been approved and ratified since the 8th ICTV Report of 2005.
Types
editPrior to 2020, enteroviruses (including all rhinoviruses) were categorized according to their serotype. In 2020 the ICTV ratified a proposal[24] to classify all new types based on the genetic diversity of their VP1 gene. Human rhinovirus type names are of the form RV-Xn where X is the rhinovirus species (A, B, or C) and n is an index number. Species A and B have used the same index up to number 100, while species C has always used a separate index. Valid index numbers are as follows:[2]
- Rhinovirus A: 1, 1B, 2, 7–13, 15, 16, 18–25, 28–34, 36, 38–41, 43–47, 49–51, 53–68, 71, 73–78, 80–82, 85, 88–90, 94–96, 100–108
- Rhinovirus B: 3–6, 14, 17, 26, 27, 35, 37, 42, 48, 52, 69, 70, 72, 79, 83, 84, 86, 91–93, 97, 99, 100-104
- Rhinovirus C: 1–57
Structure
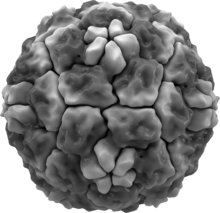
editRhinoviruses have single-stranded positive sense RNA genomes of between 7200 and 8500 nucleotides in length. At the 5' end of the genome is a virus-encoded protein and, as in mammalian mRNA, there is a 3' poly-A tail. Structural proteins are encoded in the 5' region of the genome and non structural at the 3' end. This is the same for all picornaviruses. The viral particles themselves are not enveloped and are dodecahedral in structure.
The viral proteins are translated as a single long polypeptide, which is cleaved into the structural and nonstructural viral proteins.[26]
The structure of the virus was determined in 1985 using x-ray crystallography by researchers at Purdue University and the University of Wisconsin led by Michael Rossmann. The virus was crystallized forming cubic crystals with four virus particles in each unit cell (space group P213, no. 198), similar to a cubic close-packed arrangement.[27]
Human rhinoviruses are composed of a capsid that contains four viral proteins, VP1, VP2, VP3 and VP4.[27][28] VP1, VP2, and VP3 form the major part of the protein capsid. The much smaller VP4 protein has a more extended structure, and lies at the interface between the capsid and the RNA genome. There are 60 copies of each of these proteins assembled as an icosahedron. Antibodies are a major defense against infection with the epitopes lying on the exterior regions of VP1-VP3.
Novel antiviral drugs
editThere are currently no FDA-approved antiviral drugs to treat rhinovirus infections.[5] Several novel antiviral compounds have been tested in clinical trials without sufficient efficacy to progress to FDA approval. Compounds specifically targeted for rhinoviruses, or more broadly, picornaviruses, include the following:
- Rupintrivir is a peptidomimetic drug developed for treatment of rhinovirus infections.[29] Rupintrivir inhibits human rhinovirus 3Cprotease and prevents cleavage of the rhinovirus polyprotein following translation, therefore preventing viral assembly and replication. A phase II clinical trial of rupintrivir using experimentally induced rhinovirus infection in healthy volunteers demonstrated efficacy in reducing viral load and symptom severity. However, further trials testing rupintrivir in treating natural infections showed minimal benefit, and further clinical development was halted.[30]
- Pleconaril is an orally bioavailable antiviral drug developed for the treatment of infections caused by picornaviruses.[31] This drug acts by binding to a hydrophobic pocket in VP1, and stabilizes the protein capsid to such an extent that the virus cannot release its RNA genome into the target cell. Phase III clinical trials showed a slight reduction in symptom duration if taken within 24 hours of symptom onset.[32][33] However, the FDA denied approval of pleconaril due to concerns about side effects, limited efficacy in non-white participants, and difficulty in treating most patients within a 24 hour window.[34][35]
Other treatments aiming to reduce rhinovirus infection symptoms include immunomodulatory agents. These may promote beneficial antiviral responses or reduce inflammatory responses associated with symptoms. Interferon-alpha used intranasally was shown to be effective against human rhinovirus infections. However, volunteers treated with this drug experienced some side effects, such as nasal bleeding and began developing tolerance to the drug. Subsequently, research into the treatment was abandoned.[36] Inhaled budesonide has been shown to reduce viral load and pro-inflammatory IL-1β in mice. Omalizumab, which was developed for treatment of severe allergic asthma, has shown evidence in reducing symptom severity of asthma patients infected with rhinovirus.[30]
Vaccine development
editThere are no vaccines against these viruses as there is little-to-no cross-protection between serotypes. At least 165 types of human rhinoviruses are known.[2] However, a study of the VP4 protein has shown it to be highly conserved among many serotypes of human rhinovirus, opening up the potential for a future pan-serotype human rhinovirus vaccine.[37] A similar result was obtained with the VP1 protein. Like VP4, VP1 also occasionally "pokes" out of the viral particle, making it available to neutralizing antibodies. Both peptides have been tested on rabbits, resulting in successful generation of cross-serotype antibodies.[38]
Rhinovirus genome has a high rate of variability in human circulation, even occurring with genomic sequences that differ up to 30%.[39] Recent studies have identified conserved regions of the rhinovirus genome; this, along with an adjuvanted polyvalent rhinovirus vaccine, shows potential for future development in vaccine treatment.[40]
Prevention
editHuman rhinovirus can remain infectious for up to three hours outside of a human host. Once the virus is contracted, a person is most contagious within the first three days. Preventative measures such as regular vigorous handwashing with soap and water may aid in avoiding infection. Avoiding touching the mouth, eyes, and nose (the most common entry points for rhinovirus) may also assist prevention. Droplet precautions, which take the form of a surgical mask and gloves, are the method used in major hospitals.[41] As with all respiratory pathogens once presumed to transmit via respiratory droplets, it is highly likely to be carried by the aerosols generated during routine breathing, talking, and even singing. In order to prevent airborne transmission, droplet precautions are insufficient, and routine airborne precautions are necessary.[42]
References
edit- ^ "Rhinovirus (Common Cold) | Disease Outbreak Control Division". health.hawaii.gov. Retrieved 2024-11-20.
- ^ a b c "Genus: Enterovirus | ICTV". ictv.global. Retrieved 2023-12-29.
- ^ a b Wang CC, Prather KA, Sznitman J, Jimenez JL, Lakdawala SS, Tufekci Z, et al. (August 2021). "Airborne transmission of respiratory viruses". Science. 373 (6558): eabd9149. doi:10.1126/science.abd9149. PMC 8721651. PMID 34446582.
- ^ a b c d Jacobs SE, Lamson DM, St George K, Walsh TJ (January 2013). "Human rhinoviruses". Clinical Microbiology Reviews. 26 (1): 135–162. doi:10.1128/CMR.00077-12. PMC 3553670. PMID 23297263.
- ^ a b c "Rhinoviruses: Common Colds | CDC". www.cdc.gov. 2023-03-09. Retrieved 2023-12-28.
- ^ van Benten I, Koopman L, Niesters B, Hop W, van Middelkoop B, de Waal L, et al. (2003). "Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants". Pediatric Allergy and Immunology. 14 (5): 363–370. doi:10.1034/j.1399-3038.2003.00064.x. PMC 7168036. PMID 14641606.
- ^ Auvray C, Perez-Martin S, Schuffenecker I, Pitoiset C, Tarris G, Ambert-Balay K, et al. (2024). "Sudden Infant Death Associated with Rhinovirus Infection". Viruses. 16 (4): 518. doi:10.3390/v16040518. PMC 11054477. PMID 38675861.
- ^ Friedlander SL, Busse WW (August 2005). "The role of rhinovirus in asthma exacerbations". The Journal of Allergy and Clinical Immunology. 116 (2): 267–273. doi:10.1016/j.jaci.2005.06.003. PMID 16083778.
- ^ Offit PA (2007). Vaccinated: One Man's Quest to Defeat the World's Deadliest Diseases. HarperCollins. pp. 66–68. ISBN 978-0-06-122795-0.
- ^ Public Health Reports. Vol. 74. The Service. 1959. p. 9.
- ^ Kennedy JL, Turner RB, Braciale T, Heymann PW, Borish L (June 2012). "Pathogenesis of rhinovirus infection". Current Opinion in Virology. 2 (3): 287–293. doi:10.1016/j.coviro.2012.03.008. PMC 3378761. PMID 22542099.
- ^ Andrup L, Krogfelt KA, Hansen KS, Madsen AM (August 2023). "Transmission route of rhinovirus - the causative agent for common cold. A systematic review". American Journal of Infection Control. 51 (8): 938–957. doi:10.1016/j.ajic.2022.12.005. PMID 36535318.
- ^ Kutter JS, Spronken MI, Fraaij PL, Fouchier RA, Herfst S (February 2018). "Transmission routes of respiratory viruses among humans". Current Opinion in Virology. Emerging viruses: intraspecies transmission • Viral Immunology. 28: 142–151. doi:10.1016/j.coviro.2018.01.001. PMC 7102683. PMID 29452994.
- ^ a b c d Moriyama M, Hugentobler WJ, Iwasaki A (September 2020). "Seasonality of Respiratory Viral Infections" (PDF). Annual Review of Virology. 7 (1): 83–101. doi:10.1146/annurev-virology-012420-022445. PMID 32196426. S2CID 214601321.
- ^ Fisman D (October 2012). "Seasonality of viral infections: mechanisms and unknowns". Clinical Microbiology and Infection. 18 (10): 946–954. doi:10.1111/j.1469-0691.2012.03968.x. PMID 22817528.
- ^ a b Royston L, Tapparel C (January 2016). "Rhinoviruses and Respiratory Enteroviruses: Not as Simple as ABC". Viruses. 8 (1): 16. doi:10.3390/v8010016. PMC 4728576. PMID 26761027.
- ^ Kieninger E, Fuchs O, Latzin P, Frey U, Regamey N (February 2013). "Rhinovirus infections in infancy and early childhood". The European Respiratory Journal. 41 (2): 443–452. doi:10.1183/09031936.00203511. PMID 22743674.
- ^ Palmenberg AC, Spiro D, Kuzmickas R, Wang S, Djikeng A, Rathe JA, et al. (April 2009). "Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution". Science. 324 (5923): 55–59. Bibcode:2009Sci...324...55P. doi:10.1126/science.1165557. PMC 3923423. PMID 19213880.
- ^ Bochkov YA, Watters K, Ashraf S, Griggs TF, Devries MK, Jackson DJ, et al. (April 2015). "Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication". Proceedings of the National Academy of Sciences of the United States of America. 112 (17): 5485–5490. Bibcode:2015PNAS..112.5485B. doi:10.1073/pnas.1421178112. PMC 4418890. PMID 25848009.
- ^ Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA (May 2009). "Incubation periods of acute respiratory viral infections: a systematic review". The Lancet. Infectious Diseases. 9 (5): 291–300. doi:10.1016/S1473-3099(09)70069-6. PMC 4327893. PMID 19393959.
- ^ Jackson DJ, Gern JE (March 2022). "Rhinovirus Infections and Their Roles in Asthma: Etiology and Exacerbations". The Journal of Allergy and Clinical Immunology. In Practice. 10 (3): 673–681. doi:10.1016/j.jaip.2022.01.006. PMC 10314805. PMID 35074599.
- ^ Garcia J, Espejo V, Nelson M, Sovero M, Villaran MV, Gomez J, et al. (October 2013). "Human rhinoviruses and enteroviruses in influenza-like illness in Latin America". Virology Journal. 10: 305. doi:10.1186/1743-422x-10-305. PMC 3854537. PMID 24119298.
- ^ Carstens EB, Ball LA (2009-07-01). "Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2008)". Archives of Virology. 154 (7): 1181–1188. doi:10.1007/s00705-009-0400-2. PMC 7086627. PMID 19495937.
- ^ a b Simmonds P, Gorbalenya AE, Harvala H, Hovi T, Knowles NJ, Lindberg AM, et al. (March 2020). "Recommendations for the nomenclature of enteroviruses and rhinoviruses". Archives of Virology. 165 (3): 793–797. doi:10.1007/s00705-019-04520-6. PMC 7024059. PMID 31980941.
- ^ Carstens EB (2010-01-01). "Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009)". Archives of Virology. 155 (1): 133–146. doi:10.1007/s00705-009-0547-x. PMC 7086975. PMID 19960211.
- ^ Arden KE, MacKay IM (2011). "Rhinoviruses". eLS. doi:10.1002/9780470015902.a0000431.pub3. ISBN 978-0-470-01617-6.
- ^ a b Rossmann MG, Arnold E, Erickson JW, Frankenberger EA, Griffith JP, Hecht HJ, et al. (1985). "Structure of a human common cold virus and functional relationship to other picornaviruses". Nature. 317 (6033): 145–153. Bibcode:1985Natur.317..145R. doi:10.1038/317145a0. PMID 2993920. S2CID 4288590.
- ^ Smith TJ, Kremer MJ, Luo M, Vriend G, Arnold E, Kamer G, et al. (September 1986). "The site of attachment in human rhinovirus 14 for antiviral agents that inhibit uncoating". Science. 233 (4770): 1286–1293. Bibcode:1986Sci...233.1286S. doi:10.1126/science.3018924. PMID 3018924.
- ^ Patick AK, Binford SL, Brothers MA, Jackson RL, Ford CE, Diem MD, et al. (October 1999). "In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease". Antimicrobial Agents and Chemotherapy. 43 (10): 2444–2450. doi:10.1128/AAC.43.10.2444. PMC 89498. PMID 10508022.
- ^ a b Coultas JA, Cafferkey J, Mallia P, Johnston SL (July 2021). "Experimental Antiviral Therapeutic Studies for Human Rhinovirus Infections". Journal of Experimental Pharmacology. 13: 645–659. doi:10.2147/JEP.S255211. PMC 8277446. PMID 34276229.
- ^ Pevear DC, Tull TM, Seipel ME, Groarke JM (September 1999). "Activity of pleconaril against enteroviruses". Antimicrobial Agents and Chemotherapy. 43 (9): 2109–2115. doi:10.1128/AAC.43.9.2109. PMC 89431. PMID 10471549.
- ^ Pevear DC, Hayden FG, Demenczuk TM, Barone LR, McKinlay MA, Collett MS (November 2005). "Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses". Antimicrobial Agents and Chemotherapy. 49 (11): 4492–4499. doi:10.1128/AAC.49.11.4492-4499.2005. PMC 1280128. PMID 16251287.
- ^ Fleischer R, Laessig K (December 2003). "Safety and efficacy evaluation of pleconaril for treatment of the common cold". Clinical Infectious Diseases. 37 (12): 1722. doi:10.1086/379830. PMID 14689362.
- ^ Senior K (May 2002). "FDA panel rejects common cold treatment". The Lancet. Infectious Diseases. 2 (5): 264. doi:10.1016/s1473-3099(02)00277-3. PMID 12062983.
- ^ Moynihan R, Bero L, Ross-Degnan D, Henry D, Lee K, Watkins J, et al. (June 2000). "Coverage by the news media of the benefits and risks of medications". The New England Journal of Medicine. 342 (22): 1645–1650. doi:10.1136/bmj.326.7403.1403. PMC 1126289. PMID 10833211.
- ^ Farr BM, Gwaltney JM, Adams KF, Hayden FG (July 1984). "Intranasal interferon-alpha 2 for prevention of natural rhinovirus colds". Antimicrobial Agents and Chemotherapy. 26 (1): 31–34. doi:10.1128/aac.26.1.31. PMC 179911. PMID 6089652.
- ^ Katpally U, Fu TM, Freed DC, Casimiro DR, Smith TJ (July 2009). "Antibodies to the buried N terminus of rhinovirus VP4 exhibit cross-serotypic neutralization". Journal of Virology. 83 (14): 7040–7048. doi:10.1128/JVI.00557-09. PMC 2704786. PMID 19403680.
- ^ Katpally U, Fu TM, Freed DC, Casimiro DR, Smith TJ (July 2009). "Antibodies to the buried N terminus of rhinovirus VP4 exhibit cross-serotypic neutralization". Journal of Virology. 83 (14): 7040–7048. doi:10.1128/JVI.00557-09. PMC 4291752. PMID 19403680.
- ^ Ortega H, Nickle D, Carter L (July 2021). "Rhinovirus and asthma: Challenges and opportunities". Reviews in Medical Virology. 31 (4): e2193. doi:10.1002/rmv.2193. PMC 8365703. PMID 33217098.
- ^ Makris S, Johnston S (24 September 2018). "Recent advances in understanding rhinovirus immunity". F1000Research. 7: 1537. doi:10.12688/f1000research.15337.1. PMC 6173106. PMID 30345002.
- ^ Siegel JD, Rhinehart E, Jackson M, Chiarello L (December 2007). "2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings". American Journal of Infection Control. 35 (10 Suppl 2): S65-164. doi:10.1016/j.ajic.2007.10.007. PMC 7119119. PMID 18068815.
- ^ Wang CC, Prather KA, Sznitman J, Jimenez JL, Lakdawala SS, Tufekci Z, et al. (August 2021). "Airborne transmission of respiratory viruses". Science. 373 (6558). doi:10.1126/science.abd9149. PMC 8721651. PMID 34446582.
External links
edit- VIDEO: Rhinoviruses, the Old, the New and the UW James E. Gern, MD, speaks at the University of Wisconsin School of Medicine and Public Health, 2008.
- How Big is a Human rhinovirus? (animation)