Methylscopolamine or methscopolamine, usually provided as the bromide or nitrate salt, is an oral medication used along with other medications to treat peptic ulcers by reducing stomach acid secretion.[1] Proton pump inhibitors and antihistamine medications have made this use obsolete. It can also be used for stomach or intestinal spasms, to reduce salivation, and to treat motion sickness. Methscopolamine is also commonly used as a drying agent, to dry up post-nasal drip, in cold, irritable bowel syndrome and allergy medications[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Pamine, Extendryl, AlleRx, Rescon |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606008 |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 3–4 hrs |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.314 |
| Chemical and physical data | |
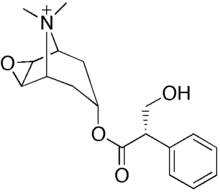
| Formula | C18H24NO4 |
| Molar mass | 318.388 g/mol (398.297 g/mol with bromide) g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Methscopolamine, a methylated derivative of scopolamine, is a muscarinic antagonist structurally similar to the neurotransmitter acetylcholine. Its mechanism of action involves blocking the muscarinic acetylcholine receptors.
It was patented in 1902 and approved for medical use in 1947.[3] Methscopolamine is an FDA-approved analog to hyoscine butylbromide.
Brand names
editBrand names include Extendryl, AlleRx, Rescon, Pamine.
References
edit- ^ Drugs.com: Methscopolamine
- ^ Gennaro AR. The Science and Practice of Pharmacy. Remington. pp. 402–403, 1025. ISBN 0912734043.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 446. ISBN 9783527607495.