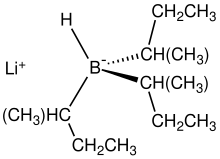
L-selectride is a organoboron compound with the chemical formula Li[(CH3CH2CH(CH3))3BH]. A colorless salt, it is usually dispensed as a solution in THF. As a particularly basic and bulky borohydride, it is used for stereoselective reduction of ketones.[1] [1]
| |
| Names | |
|---|---|
| IUPAC name
lithium tri-sec-butyl(hydrido)borate(1-)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.049.166 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H28BLi | |
| Molar mass | 190.10 g/mol |
| Appearance | Colorless liquid |
| Density | 0.870 g/ml |
| Reacts with water | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Water reactive, flammable, burns skin and eyes |
| Flash point | -17 °F |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Use in synthesis
editLike other borohydrides, reductions are effected in two steps: delivery of the hydride equivalent to give the lithium alkoxide followed by hydrolytic workup:
- R2CO + Li[(CH3CH2CH(CH3))3BH] → R2CHOLi + (CH3CH2CH(CH3))3B
- R2CHOLi + H2O → R2CH2OH + LiOH
The selectivity of this reagent is illustrated by its reduction of all three methylcyclohexanones to the less stable methylcyclohexanols in >98% yield.
Under certain conditions, L-selectride can selectively reduce enones by conjugate addition of hydride, owing to the greater steric hindrance the bulky hydride reagent experiences at the carbonyl carbon relative to the (also-electrophilic) β-position.[2] L-Selectride can also stereoselectively reduce carbonyl groups in a 1,2-fashion, again due to the steric nature of the hydride reagent.[3]
It reduces ketones to alcohols.[4]
Related compounds
editN-selectride and K-selectride are related compounds, but instead of lithium as cation they have sodium and potassium cations respectively. These reagents can sometimes be used as alternatives to, for instance, sodium amalgam reductions in inorganic chemistry.[5]
Related compounds
edit- Lithium Trisiamylborohydride[6]
- Lithium triethylborohydride ("Super hydride")
References
edit- ^ a b Hubbard, John L.; Dake, Gregory (2012). "Lithium Tri- sec -butylborohydride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rl145.pub2. ISBN 978-0-471-93623-7.
- ^ Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (1st ed.). Oxford University Press. p. 685. ISBN 978-0-19-850346-0.
- ^ Scott A. Miller and A. Richard Chamberlin (1989). "Highly selective formation of cis-substituted hydroxylactams via auxiliary-controlled reduction of imides". J. Org. Chem. 54 (11): 2502–2504. doi:10.1021/jo00272a004.
- ^ S. D. Knight, L. E. Overman and G. Pairaudeau (1993). "Synthesis applications of cationic aza-Cope rearrangements. 26. Enantioselective total synthesis of (−)-strychnine". J. Am. Chem. Soc. 115 (20): 9293–9294. doi:10.1021/ja00073a057.
- ^ Gladysz, J. A.; Williams, G. M.; Tam, Wilson; Johnson, Dennis Lee; Parker, David W.; Selover, J. C. (1979). "Synthesis of metal carbonyl monoanions by trialkylborohydride cleavage of metal carbonyl dimers: A convenient one-flask preparation of metal alkyls, metal acyls, and mixed-metal compounds". Inorganic Chemistry. 18 (3): 553–558. doi:10.1021/ic50193a006.
- ^ Zaidlewicz, Marek; Brown, Herbert C. (2001). "Lithium Trisiamylborohydride". Encyclopedia of Reagents for Organic Synthesis (EROS). doi:10.1002/047084289X.rl151. ISBN 0-471-93623-5.