Isopregnanolone, also known as isoallopregnanolone and epiallopregnanolone, as well as sepranolone (INN), and as 3β-hydroxy-5α-pregnan-20-one or 3β,5α-tetrahydroprogesterone (3β,5α-THP), is an endogenous neurosteroid and a natural 3β-epimer of allopregnanolone.[1][2] It has been reported to act as a subunit-selective negative allosteric modulator of the GABAA receptor,[2] and antagonizes in animals and humans some but not all of the GABAA receptor-mediated effects of allopregnanolone, such as anesthesia,[3] sedation,[4] and reduced saccadic eye movements,[4] but not learning impairment.[2] Isopregnanolone has no hormonal effects and appears to have no effect on the GABAA receptor by itself; it selectively antagonizes allopregnanolone and does not affect the effects of other types of GABAA receptor positive allosteric modulators such as benzodiazepines or barbiturates.[1][5]
| |
| Names | |
|---|---|
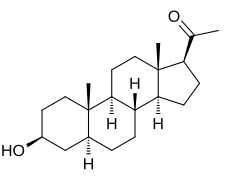
| IUPAC name
3β-Hydroxy-5α-pregnan-20-one
| |
| Systematic IUPAC name
1-[(1S,3aS,3bR,5aS,7S,9aS,9bS,11aS)-7-Hydroxy-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-1-yl]ethan-1-one | |
| Other names
Isoallopregnanolone; Epiallopregnanolone; Sepranolone; 3β,5α-Tetrahydroprogesterone; 3β,5α-THP
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.478 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H34O2 | |
| Molar mass | 318.49 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isopregnanolone is synthesized from progesterone in the body by the actions of the enzymes 5α-reductase and 3β-hydroxysteroid dehydrogenase (with 5α-dihydroprogesterone as the intermediate in this two-step transformation)[6] and can be reversibly metabolized into allopregnanolone by the enzyme 3α-hydroxysteroid dehydrogenase.[1][2] Levels of isopregnanolone, progesterone, and allopregnanolone are highly correlated across the menstrual cycle and throughout pregnancy.[1] The concentrations of isopregnanolone are significantly less than those of progesterone and allopregnanolone; about half of those of allopregnanolone, to be precise.[6] Isopregnanolone has a relatively long serum elimination half-life of 14 hours in humans.[1]
Isopregnanolone (developmental code name UC-1010) is under development for the treatment of premenstrual dysphoric disorder.[7][8] As of 2017, it is in phase II clinical trials for this indication.[7][8]
Chemistry
editSee also
editReferences
edit- ^ a b c d e Hedström H, Bixo M, Nyberg S, Spigset O, Zingmark E, Bäckström T (2009). "Studies of pharmacokinetic and pharmacodynamic properties of isoallopregnanolone in healthy women". Psychopharmacology. 203 (1): 85–98. doi:10.1007/s00213-008-1372-8. PMID 18949461. S2CID 720976.
- ^ a b c d Öfverman, C., Strömberg, J., Birzniece, V., Turkmen, S., Hill, M., Lundgren, P., ... & Johansson, I. M. (2009). The progesterone metabolite isoallopregnanolone is a subunit-selective antagonist of the GABA-A receptor. Chicago
- ^ Bäckström T, Wahlström G, Wahlström K, Zhu D, Wang MD (2005). "Isoallopregnanolone; an antagonist to the anaesthetic effect of allopregnanolone in male rats". Eur. J. Pharmacol. 512 (1): 15–21. doi:10.1016/j.ejphar.2005.01.049. PMID 15814085.
- ^ a b Bengtsson SK, Nyberg S, Hedström H, Zingmark E, Jonsson B, Bäckström T, Bixo M (2015). "Isoallopregnanolone antagonize allopregnanolone-induced effects on saccadic eye velocity and self-reported sedation in humans". Psychoneuroendocrinology. 52: 22–31. doi:10.1016/j.psyneuen.2014.10.025. PMID 25459890. S2CID 25703203.
- ^ Lundgren, Per; Strömberg, Jessica; Bäckström, Torbjörn; Wang, Mingde (2003). "Allopregnanolone-stimulated GABA-mediated chloride ion flux is inhibited by 3β-hydroxy-5α-pregnan-20-one (isoallopregnanolone)". Brain Research. 982 (1): 45–53. doi:10.1016/S0006-8993(03)02939-1. ISSN 0006-8993. PMID 12915239. S2CID 54297803.
- ^ a b Abraham Weizman (1 February 2008). Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders: Novel Strategies for Research and Treatment. Springer Science & Business Media. pp. 8–9. ISBN 978-1-4020-6854-6.
- ^ a b "Sepranolone - Asarina Pharma - AdisInsight".
- ^ a b Bixo M, Ekberg K, Poromaa IS, Hirschberg AL, Jonasson AF, Andréen L, Timby E, Wulff M, Ehrenborg A, Bäckström T (2017). "Treatment of premenstrual dysphoric disorder with the GABAA receptor modulating steroid antagonist Sepranolone (UC1010)-A randomized controlled trial". Psychoneuroendocrinology. 80: 46–55. doi:10.1016/j.psyneuen.2017.02.031. PMID 28319848.