Inflammatory myopathy, also known as idiopathic inflammatory myopathy (IIM), is disease featuring muscle weakness, inflammation of muscles (myositis), and in some types, muscle pain (myalgia). The cause of much inflammatory myopathy is unknown (idiopathic), and such cases are classified according to their symptoms and signs, electromyography, MRI, and laboratory findings. It can also be associated with underlying cancer. The main classes of idiopathic inflammatory myopathy are polymyositis (PM), dermatomyositis (DM) (including juvenile, amyopathic, and sine-dermatitis form), inclusion-body myositis (IBM), immune-mediated necrotising myopathy (IMNM), and focal autoimmune myositis.[1]
| Inflammatory myopathy | |
|---|---|
| Other names | Idiopathic inflammatory myopathy |
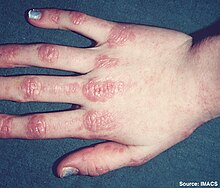 | |
| Gottron's papules on the hand of a patient with juvenile dermatomyositis | |
| Specialty | Rheumatology |
Diagnosis
editIdiopathic inflammatory myopathy is a diagnosis of exclusion. There are a number of known causes of myopathy, and it is only once these have been ruled out that a clinician should assign an idiopathic inflammatory myopathy. The usual criteria for a diagnosis of PM are weakness in muscles of the head, neck, trunk, upper arms or upper legs; raised blood serum concentrations of some muscle enzymes such as creatine kinase; unhealthy muscle changes on electromyography; and biopsy findings of (i) muscle cell degeneration and regeneration and (ii) chronic inflammatory infiltrates in muscle cells. If heliotrope (purple) rash or Gottron's papules are also present, then the diagnosis is DM. In DM, myositis may not be clinically apparent but detectable via biopsy or MRI.[2] If the criteria for PM are met but muscle weakness also affects the hands and feet or is not accompanied by pain IBM should be suspected, and confirmed when muscle cell biopsy reveals (i) cytoplasmic vacuoles fringed by basophilic granules and (ii) inflammatory infiltrate comprising mostly CD8 T lymphocytes and macrophages; and electron microscopy reveals filamentous inclusions in both cytoplasm and nucleus.[3]
Treatment
editThere have been few randomized treatment trials, due to the relative rarity of inflammatory myopathies.[4] The goal of treatment is improvement in activities of daily living and muscle strength. Suppression of immune system activity (immunosuppression) is the treatment strategy. Patients with PM or DM almost always improve to some degree in response to treatment, at least initially, and many recover fully with maintenance therapy. (If there is no initial improvement from treatment of PM or DM, the diagnosis should be carefully re-examined.) There is no proven effective therapy for IBM, and most IBM patients will need assistive devices such as a cane, a walking frame or a wheelchair. The later in life IBM arises, the more aggressive it appears to be.[5]
Polymyositis and dermatomyositis
editIn severe cases of PM and DM with systemic signs, an initial three to five days on intravenous corticosteroid (methylprednisolone) may be used; but normally treatment begins with a single daily (after breakfast) high dose of oral corticosteroid (prednisone). After a month or so the strength of every second day's dose is very gradually reduced over three to four months, to minimize the negative effects of the prednisone. When a high dose of prednisone cannot be reduced without losing muscle strength, or when prednisone is effective but it is producing significant complications, "steroid sparing" oral immunosuppressants such as azathioprine, mycophenolate mofetil, methotrexate and cyclosporine, may be used in combination with reduced prednisone. Some of these steroid sparing drugs can take several months to demonstrate an effect.[5]
To minimize side effects, patients on corticosteroids should follow a strict high-protein, low-carbohydrate, low-salt diet; and with long-term corticosteroid use a daily calcium supplement and weekly vitamin D supplement (and a weekly dose of Fosamax for postmenopausal women) should be considered.[5]
For patients not responding to this approach there is weak evidence supporting the use of intravenous immunoglobulin, ciclosporin, tacrolimus, mycophenolate mofetil and other agents; and trials of rituximab have indicated a potential therapeutic effect.[4]
Inclusion-body myositis
editDespite its very similar clinical presentation to PM, IBM does not respond to the drugs that effectively treat PM, and there is no proven effective therapy for IBM.[5] Alemtuzumab is being studied but as of May 2013 it had not demonstrated clinical effectiveness in IBM.[4] Dysphagia (difficulty swallowing) may be improved by intravenous immunoglobulin, though more trials are needed.[6] Non-fatiguing, systematic strength-building exercise has demonstrated benefit.[7] Occupational and rehabilitation therapists can offer good advice on walking without falling and performing fine motor tasks, and can provide appropriate canes, braces and wheelchairs. Speech pathologists can provide advice on preventing choking episodes and reducing the anxiety of an immanent aspiration for both patients and carers.[5]
Epidemiology
editEvery year between 2.18 and 7.7 people per million receive a diagnosis of PM or DM.[8] Around 3.2 children per million per year are diagnosed with DM (termed juvenile dermatomyositis), with an average age of onset of seven years. Diagnosis of adult DM commonly occurs between 30 and 50 years of age. PM is an adult disease, usually emerging after the age of twenty. PM and DM are more common in females, more common in Caucasians, and least common in Asians. At any given time, about 35.5 people per million have IBM; it emerges after the age of 30 (usually after 50), and may be more common in males.[4]
Differential diagnoses
editMyositis (inflammation of the muscle) can be caused secondary to a number of primary diseases including: infection, muscle trauma, medications and toxins, inherited muscle diseases, and autoimmune disease such as lupus. Other autoimmune diseases can mimic the symptoms of IMM (muscle weakness and autoantibodies), including Lambert–Eaton myasthenic syndrome, myasthenia gravis, and the muscle form of sarcoidosis.[1]
Although idiopathic inflammatory myopathy (IIM) is a diagnosis of exclusion, it is often the initial misdiagnosis of several acquired non-inflammatory myopathies.[1][9][10] This is due to a number of factors, including:
- overlapping symptoms (such as muscle weakness, pain, elevated CK);
- that delaying treatment for an inflammatory myopathy, in order to exclude potential non-inflammatory myopathies, may cause irreversible damage (although administering immunosuppressants and glucocorticosteroids to non-inflammatory myopathies may also cause damage);
- that many of the testing methods for idiopathic inflammatory myopathy are non-specific (the same results could apply to many different myopathies);
- and the unfamiliarity of the many rare diseases that are outside of rheumatology.[1][10]
Acquired non-inflammatory myopathies include toxin-induced myopathies, endocrine myopathies, metabolic myopathies, muscular dystrophies, congenital myasthenic syndrome, nutritional imbalances, and infection.[11][1]
See also
editReferences
edit- ^ a b c d e Szczęsny P, Świerkocka K, Olesińska M (2018). "Differential diagnosis of idiopathic inflammatory myopathies in adults - the first step when approaching a patient with muscle weakness". Reumatologia. 56 (5): 307–315. doi:10.5114/reum.2018.79502. PMC 6263305. PMID 30505013.
- ^ Miller FW (10 June 2009). "Classification of Idiopathic Inflammatory Myopathies". In Kagen LJ (ed.). The Inflammatory Myopathies. Springer. pp. 23–36. ISBN 9781627038393.
- ^ Kagen LJ (10 June 2009). "Inclusion Body Myositis". In Kagen LJ (ed.). The Inflammatory Myopathies. Springer. pp. 95–102. ISBN 9781627038393.
- ^ a b c d Holton JL, Wedderburn LR, Hanna MG (29 May 2013). "Polymyositis, Dermatomyositis and Inclusion Body Myositis". In Goebel HH, Sewry CA, Weller RO (eds.). Muscle Disease: Pathology and Genetics. John Wiley & Sons. pp. 747–781. ISBN 978-1-118-63549-0.
- ^ a b c d e Dalakas MC (8 September 2010). "Treatment and Management of Autoimmune Myopathies". In Bertorini TE (ed.). Neuromuscular Disorders. Elsevier Health Sciences. pp. 395–408. ISBN 9781437703726.
- ^ Cited in Dalakas, Marinos C (08 September 2010). "Treatment and Management of Autoimmune Myopathies". In Bertorini, Tulio E. Neuromuscular Disorders. Elsevier Health Sciences. pp. 395—408. ISBN 9781437703726:
- Dalakas MC, Sonies B, Dambrosia J, Sekul E, Cupler E, Sivakumar K (March 1997). "Treatment of inclusion-body myositis with IVIg: a double-blind, placebo-controlled study". Neurology. 48 (3): 712–716. doi:10.1212/wnl.48.3.712. PMID 9065553. S2CID 25118314.
- Walter MC, Lochmüller H, Toepfer M, Schlotter B, Reilich P, Schröder M, et al. (January 2000). "High-dose immunoglobulin therapy in sporadic inclusion body myositis: a double-blind, placebo-controlled study". Journal of Neurology. 247 (1): 22–28. doi:10.1007/s004150050005. PMID 10701893. S2CID 23409201.
- Cherin P, Pelletier S, Teixeira A, Laforet P, Simon A, Herson S, Eymard B (January 2002). "Intravenous immunoglobulin for dysphagia of inclusion body myositis". Neurology. 58 (2): 326. doi:10.1212/wnl.58.2.326. PMID 11805271. S2CID 40526855.
- ^ Spector SA, Lemmer JT, Koffman BM, Fleisher TA, Feuerstein IM, Hurley BF, Dalakas MC (October 1997). "Safety and efficacy of strength training in patients with sporadic inclusion body myositis". Muscle & Nerve. 20 (10): 1242–1248. doi:10.1002/(sici)1097-4598(199710)20:10<1242::aid-mus6>3.3.co;2-x. PMID 9324080. Cited in:
- Dalakas, Marinos C (08 September 2010). "Treatment and Management of Autoimmune Myopathies". In Bertorini, Tulio E. Neuromuscular Disorders. Elsevier Health Sciences. pp. 395—408. ISBN 9781437703726
- ^ Mastaglia FL, Garlepp MJ, Phillips BA, Zilko PJ (April 2003). "Inflammatory myopathies: clinical, diagnostic and therapeutic aspects". Muscle & Nerve. 27 (4): 407–425. doi:10.1002/mus.10313. PMID 12661042. S2CID 24978554. Cited in:
- Holton JL, Wedderburn LR, Hanna MG (29 May 2013). "Polymyositis, Dermatomyositis and Inclusion Body Myositis". In Goebel HH, Sewry CA, Weller RO (eds.). Muscle Disease: Pathology and Genetics. John Wiley & Sons. pp. 747–781. ISBN 978-1-118-63549-0.
- ^ Tarnopolsky MA, Hatcher E, Shupak R (May 2016). "Genetic Myopathies Initially Diagnosed and Treated as Inflammatory Myopathy". The Canadian Journal of Neurological Sciences. Le Journal Canadien des Sciences Neurologiques. 43 (3): 381–384. doi:10.1017/cjn.2015.386. PMID 26911292. S2CID 25515951.
- ^ a b Bhai SF, Dimachkie MM, de Visser M (June 2022). "Is it really myositis? Mimics and pitfalls". Best Practice & Research. Clinical Rheumatology. 36 (2): 101764. doi:10.1016/j.berh.2022.101764. PMID 35752578. S2CID 250012951.
- ^ Baer AN, Wortmann RL (May 2013). "Noninflammatory myopathies". Rheumatic Disease Clinics of North America. 39 (2): 457–479. doi:10.1016/j.rdc.2013.02.006. PMID 23597974.