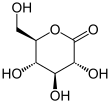
Glucono-δ-lactone (GDL), also known as gluconolactone, is an organic compound with the formula (HOCH)3(HOCH2CH)CO2. A colorless solid, it is an oxidized derivative of glucose.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
D-Glucono-1,5-lactone
| |||
| Systematic IUPAC name
(3R,4S,5S,6R)-3,4,5-Trihydroxy-6-(hydroxymethyl)oxan-2-one[2] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.001.833 | ||
| EC Number |
| ||
| E number | E575 (acidity regulators, ...) | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H10O6 | |||
| Molar mass | 178.140 g·mol−1 | ||
| Melting point | 150–153 °C (302–307 °F; 423–426 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
It is typically produced by the aerobic oxidation of glucose in the presence of the enzyme glucose oxidase. The conversion cogenerates hydrogen peroxide, which is often the key product of the enzyme:
- C6H12O6 + O2 → C6H10O6 + H2O2
Gluconolactone spontaneously hydrolyzes to gluconic acid:[4]
- C6H10O6 + H2O → C6H12O7
Applications
editGluconolactone is a food additive with the E-number E575[5] used as a sequestrant, an acidifier,[6] or a curing, pickling, or leavening agent. It is a lactone of D-gluconic acid. Pure GDL is a white odorless crystalline powder.
GDL has been marketed for use in feta cheese.[7] GDL is pH-neutral, but hydrolyses in water to gluconic acid which is acidic, adding a tangy taste to foods, though it has roughly a third of the sourness of citric acid. It is metabolized to 6-phospho-D-gluconate; one gram of GDL yields roughly the same amount of metabolic energy as one gram of sugar.
Upon addition to water, GDL is partially hydrolysed to gluconic acid, with the balance between the lactone form and the acid form established as a chemical equilibrium. The rate of hydrolysis of GDL is increased by heat and high pH.[8]
The yeast Maudiozyma bulderi can be used to ferment gluconolactone to ethanol and carbon dioxide. The pH value greatly affects culture growth. Gluconolactone at 1 or 2% in a mineral media solution causes the pH to drop below 3.[9]
It is also a complete inhibitor of the enzyme amygdalin beta-glucosidase at concentrations of 1 mM.[10]
See also
editReferences
edit- ^ Budavari, Susan, ed. (2001), The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (13th ed.), Merck, ISBN 0911910131, 4469.
- ^ a b PubChem. "D-Gluconic acid, delta-lactone". pubchem.ncbi.nlm.nih.gov. Archived from the original on 2022-02-25. Retrieved 2022-05-03.
- ^ Beil. 18, V, 5, 11
- ^ Wong, Chun Ming; Wong, Kwun Hei; Chen, Xiao Dong (2008). "Glucose oxidase: Natural Occurrence, Function, Properties and Industrial Applications". Applied Microbiology and Biotechnology. 78 (6): 927–938. doi:10.1007/s00253-008-1407-4. PMID 18330562. S2CID 2246466.
- ^ "Current EU approved additives and their E Numbers". Food Standards Agency. Archived from the original on 22 April 2022.
- ^ Martin, F.; Cayot, N.; Marin, A.; et al. (2009). "Effect of oxidoreduction potential and of gas bubbling on rheological properties and microstructure of acid skim milk gels acidified with glucono-δ-lactone" (PDF). Journal of Dairy Science. 92 (12): 5898–5906. doi:10.3168/jds.2009-2491. PMID 19923593. Archived (PDF) from the original on 2020-03-11. Retrieved 2019-08-16.
- ^ Blythman, Joanna (21 February 2015). "Inside the food industry: the surprising truth about what you eat". The Guardian. Archived from the original on 13 August 2016. Retrieved 28 October 2016.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ^ Pocker, Y.; Green, Edmond (1973). "Hydrolysis of D-Glucono-δ-lactone. I. General Acid–Base Catalysis, Solvent Deuterium Isotope Effects, and Transition State Characterization". J. Am. Chem. Soc. 95 (1): 113–19. doi:10.1021/ja00782a019. PMID 4682891.
- ^ Van Dijken, J. P.; Van Tuijl, A.; Luttik, M. A.; Middelhoven, W. J.; Pronk, J. T. (2002). "Novel pathway for alcoholic fermentation of delta-gluconolactone in the yeast Saccharomyces bulderi". Journal of Bacteriology. 184 (3): 672–678. doi:10.1128/JB.184.3.672-678.2002. PMC 139522. PMID 11790736.
- ^ Petruccioli, M.; Brimer, L.; Cicalini, A. R.; Federici, F. (1999). "Production and Properties of the Linamarase and Amygdalase Activities of Penicillium aurantiogriseum P35". Bioscience, Biotechnology, and Biochemistry. 63 (5): 805–812. doi:10.1271/bbb.63.805. PMID 10380623.