Glucagon-like peptide-1 (GLP-1) is a 30- or 31-amino-acid-long peptide hormone deriving from the tissue-specific posttranslational processing of the proglucagon peptide. It is produced and secreted by intestinal enteroendocrine L-cells and certain neurons within the nucleus of the solitary tract in the brainstem upon food consumption. The initial product GLP-1 (1–37) is susceptible to amidation and proteolytic cleavage, which gives rise to the two truncated and equipotent biologically active forms, GLP-1 (7–36) amide and GLP-1 (7–37). Active GLP-1 protein secondary structure includes two α-helices from amino acid position 13–20 and 24–35 separated by a linker region.
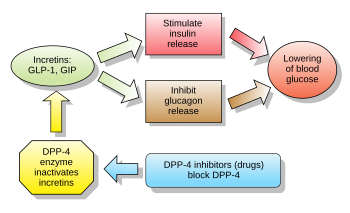
Alongside glucose-dependent insulinotropic peptide (GIP), GLP-1 is an incretin; thus, it has the ability to decrease blood sugar levels in a glucose-dependent manner by enhancing the secretion of insulin. Beside the insulinotropic effects, GLP-1 has been associated with numerous regulatory and protective effects. Unlike GIP, the action of GLP-1 is preserved in patients with type 2 diabetes. Glucagon-like peptide-1 receptor agonists gained approval as drugs to treat diabetes and obesity starting in the 2000s.
Endogenous GLP-1 is rapidly degraded primarily by dipeptidyl peptidase-4 (DPP-4), as well as neutral endopeptidase 24.11 (NEP 24.11) and renal clearance, resulting in a half-life of approximately 2 minutes. Consequently, only 10–15 % of GLP-1 reaches circulation intact, leading to fasting plasma levels of only 0–15 pmol/L. To overcome this, GLP-1 receptor agonists and DPP-4 inhibitors have been developed to increase GLP-1 activity. As opposed to common treatment agents such as insulin and sulphonylurea, GLP-1-based treatment has been associated with weight loss and a lower risk of hypoglycemia, two important considerations for patients with type 2 diabetes.
Gene expression
editThe proglucagon gene is expressed in several organs including the pancreas (α-cells of the islets of Langerhans), gut (intestinal enteroendocrine L-cells) and brain (caudal brainstem and hypothalamus). Pancreatic proglucagon gene expression is promoted upon fasting and hypoglycaemia induction and inhibited by insulin. Conversely, intestinal proglucagon gene expression is reduced during fasting and stimulated upon food consumption. In mammals, the transcription gives rise to identical mRNA in all three cell types, which is further translated to the 180 amino acid precursor called proglucagon. However, as a result of tissue-specific posttranslational processing mechanisms, different peptides are produced in the different cells.[1][2]
In the pancreas (α-cells of the islets of Langerhans), proglucagon is cleaved by prohormone convertase (PC) 2 producing glicentin-related pancreatic peptide (GRPP), glucagon, intervening peptide-1 (IP-1) and major proglucagon fragment (MPGF).[3]
In the gut and brain, proglucagon is catalysed by PC 1/3 giving rise to glicentin, which may be further processed to GRPP and oxyntomodulin, GLP-1, intervening peptide-2 (IP-2) and glucagon-like peptide-2 (GLP-2). Initially, GLP-1 was thought to correspond to proglucagon (72–108) suitable with the N-terminal of the MPGF, but sequencing experiments of endogenous GLP-1 revealed a structure corresponding to proglucagon (78–107) from which two discoveries were found. Firstly, the full-length GLP-1 (1–37) was found to be catalysed by endopeptidase to the biologically active GLP-1 (7–37). Secondly, the glycine corresponding to proglucagon(108) was found to serve as a substrate for amidation of the C-terminal arginine resulting in the equally potent GLP-1 (7–36) amide. In humans, almost all (>80%) secreted GLP-1 is amidated, whereas a considerable part remains GLP-1 (7–37) in other species.[3][4]
Secretion
editGLP-1 is packaged in secretory granules and secreted into the hepatic portal system by the intestinal L-cells located primarily in the distal ileum and colon, but also found in the jejunum and duodenum. The L-cells are open-type triangular epithelial cells directly in contact with the lumen and neuro-vascular tissue and are accordingly stimulated by various nutrient, neural and endocrine factors.[2]
GLP-1 is released in a biphasic pattern with an early phase after 10–15 minutes followed by a longer second phase after 30–60 minutes upon meal ingestion. As the majority of L-cells are located in the distal ileum and colon, the early phase is likely explained by neural signalling, gut peptides or neurotransmitters. Other evidence suggest that the amount of L-cells located in the proximal jejunum is sufficient to account for the early phase secretion through direct contact with luminal nutrients. Less controversially, the second phase is likely caused by direct stimulation of L-cells by digested nutrients. The rate of gastric emptying is therefore an important aspect to consider, as it regulates the entry of nutrients into the small intestines where the direct stimulation occurs. One of the actions of GLP-1 is to inhibit gastric emptying, thus slowing down its own secretion upon postprandial activation.[1][2]
Fasting plasma concentration of biologically active GLP-1 range between 0 and 15 pmol/L in humans and is increased 2- to 3-fold upon food consumption depending on meal size and nutrient composition. Individual nutrients, such as fatty acids, essential amino acids and dietary fibre have also shown to stimulate GLP-1 secretion.
Sugars have been associated with various signalling pathways, which initiate depolarisation of the L-cell membrane causing an elevated concentration of cytosolic Ca2+ which in turn induce GLP-1 secretion. Fatty acids have been associated with the mobilisation of intracellular Ca2+ stores and subsequently release of Ca2+ into the cytosol. The mechanisms of protein-triggered GLP-1 secretion are less clear, but the amino acid proportion and composition appear important to the stimulatory effect.[5]
Degradation
editOnce secreted, GLP-1 is extremely susceptible to the catalytic activity of the proteolytic enzyme dipeptidyl peptidase-4 (DPP-4). Specifically, DPP-4 cleaves the peptide bond between Ala8-Glu9 resulting in the abundant GLP-1 (9–36) amide constituting 60–80 % of total GLP-1 in circulation. DPP-4 is widely expressed in multiple tissues and cell types and exists in both a membrane-anchored and soluble circulating form. Notably, DPP-4 is expressed on the surface of endothelial cells, including those located directly adjacent to GLP-1 secretion sites.[2] Consequently, less than 25% of secreted GLP-1 is estimated to leave the gut intact. Additionally, presumably due to the high concentration of DPP-4 found on hepatocytes, 40–50% of the remaining active GLP-1 is degraded across the liver. Thus, due to the activity of DPP-4 only 10–15 % of secreted GLP-1 reaches circulation intact.[3]
Neutral endopeptidase 24.11 (NEP 24.11) is a membrane-bound zinc metallopeptidase widely expressed in several tissues, but found in particularly high concentrations in the kidneys, which is also identified accountable for the rapid degradation of GLP-1. It primarily cleaves peptides at the N-terminal side of aromatic amino acids or hydrophobic amino acids and is estimated to contribute by up to 50% of the GLP-1 degradation. However, the activity only becomes apparent once the degradation of DPP-4 has been prevented, as the majority of GLP-1 reaching the kidneys have already been processed by DPP-4. Similarly, renal clearance appear more significant for the elimination of already inactivated GLP-1.[6]
The resulting half-life of active GLP-1 is approximately 2 minutes, which is however sufficient to activate GLP-1 receptors.
Physiological functions
editGLP-1 possesses several physiological properties making it (and its functional analogs) a subject of intensive investigation as a potential treatment of diabetes mellitus, as these actions induce long-term improvements along with the immediate effects.[need quotation to verify][7][8][9][10] Although reduced GLP-1 secretion has previously been associated with attenuated incretin effect in patients with type 2 diabetes, it is now granted that GLP-1 secretion in patients with type 2 diabetes does not differ from healthy subjects.[11]
The most noteworthy effect of GLP-1 is its ability to promote insulin secretion in a glucose-dependent manner. As GLP-1 binds to GLP-1 receptors expressed on the pancreatic β cells, the receptors couple to G-protein subunits and activate adenylate cyclase that increases the production of cAMP from ATP.[3] Subsequently, activation of secondary pathways, including PKA and Epac2, alters the ion channel activity causing elevated levels of cytosolic Ca2+ that enhances exocytosis of insulin-containing granules. During the process, influx of glucose ensures sufficient ATP to sustain the stimulatory effect.[3]
Additionally, GLP-1 ensures the β cell insulin stores are replenished to prevent exhaustion during secretion by promoting insulin gene transcription, mRNA stability and biosynthesis.[2][12] GLP-1 evidently also increases[13] β cell mass by promoting proliferation and neogenesis while inhibiting apoptosis. As both type 1 and 2 diabetes are associated with reduction of functional β cells, this effect is highly interesting regarding diabetes treatment.[12] Considered almost as important as the effect of enhancing insulin secretion, GLP-1 has been shown to inhibit glucagon secretion at glucose levels above fasting levels. Critically, this does not affect the glucagon response to hypoglycemia as this effect is also glucose-dependent. The inhibitory effect is presumably mediated indirectly through somatostatin secretion, but a direct effect cannot be completely excluded.[14][15]
In the brain, GLP-1 receptor activation has been linked with neurotrophic effects including neurogenesis[16][17] and neuroprotective effects including reduced necrotic[18] and apoptotic[19][18] signaling, cell death,[20][21] and dysfunctions.[22] In the diseased brain, GLP-1 receptor agonist treatment is associated with protection against a range of experimental disease models such as Parkinson's disease,[23][17] Alzheimer's disease,[24][25] stroke,[23] traumatic brain injury,[13][18] and multiple sclerosis.[26] In accordance with the expression of GLP-1 receptor on brainstem and hypothalamus, GLP-1 has been shown to promote satiety and thereby reduce food and water intake. Consequently, diabetic subjects treated with GLP-1 receptor agonists often experience weight loss as opposed to the weight gain commonly induced with other treatment agents.[2][15]
In the stomach, GLP-1 inhibits gastric emptying, acid secretion and motility, which collectively decrease appetite. By decelerating gastric emptying GLP-1 reduces postprandial glucose excursion which is another attractive property regarding diabetes treatment. However, these gastrointestinal activities are also the reason why subjects treated with GLP-1-based agents occasionally experience nausea.[14]
GLP-1 has also shown signs of carrying out protective and regulatory effects in numerous other tissues, including heart, tongue, adipose, muscles, bones, kidneys, liver and lungs.
Research history
editIn the early 1980s, Richard Goodman and P. Kay Lund were postdoctoral researchers working in Joel Habener's laboratory at Massachusetts General Hospital.[27] Starting in 1979, Goodman harvested DNA from American anglerfish islet cells and spliced the DNA into bacteria to find the gene for somatostatin, then Lund joined the Habener lab and used Goodman's bacteria to search for the gene for glucagon.[27] In 1982, they published their discovery that the gene for proglucagon actually codes for three peptides: glucagon and two novel peptides.[27] Those two novel peptides were later isolated, identified, and investigated by other researchers, and are now known as glucagon-like peptide-1 and glucagon-like peptide-2.[27]
In the 1980s, Svetlana Mojsov worked on the identification of GLP-1 at Mass General, where she was head of a peptide synthesis facility.[28] To try to identify whether a specific fragment of GLP-q was an incretin, Mojsov created an incretin-antibody and developed ways to track its presence. She identified that a stretch of 31 amino acids in the GLP-1 was an incretin.[29][30] Mojsov and her collaborators Daniel J. Drucker and Habener showed that small quantities of lab-synthesized GLP-1 could trigger insulin.[31][32][33]
Mojsov fought to have her name included in patents, with Mass General eventually agreeing to amend four patents to include her name. She received her one-third of drug royalties for one year.[34]
The discovery of GLP-1's extremely short half-life meant that it was impossible to develop into a drug.[35][36] This caused diabetes research to shift towards other therapeutic options such as targeting the GLP-1 receptor, which then led to the development of GLP-1 receptor agonists.[35][36]
See also
edit- Glucagon
- Glucagon-like peptide 1 receptor
- Glucagon-like peptide-2
- Type 2 diabetes
- GLP-1 receptor agonists : albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, tirzepatide and semaglutide, the latter of which is marketed under the brands Ozempic, Rybelsus and Wegovy.
- Dipeptidyl peptidase-4
- Glucose-dependent insulinotropic peptide
References
edit- ^ a b Marathe CS, Rayner CK, Jones KL, Horowitz M (June 2013). "Glucagon-like peptides 1 and 2 in health and disease: a review". Peptides. 44: 75–86. doi:10.1016/j.peptides.2013.01.014. PMID 23523778. S2CID 22641629.
- ^ a b c d e f Baggio LL, Drucker DJ (May 2007). "Biology of incretins: GLP-1 and GIP". Gastroenterology. 132 (6): 2131–57. doi:10.1053/j.gastro.2007.03.054. PMID 17498508.
- ^ a b c d e Holst JJ (October 2007). "The physiology of glucagon-like peptide 1". Physiological Reviews. 87 (4): 1409–39. doi:10.1152/physrev.00034.2006. PMID 17928588.
- ^ Deacon CF, Holst JJ (August 2009). "Immunoassays for the incretin hormones GIP and GLP-1". Best Practice & Research. Clinical Endocrinology & Metabolism. 23 (4): 425–32. doi:10.1016/j.beem.2009.03.006. PMID 19748060.
- ^ Ma X, Guan Y, Hua X (September 2014). "Glucagon-like peptide 1-potentiated insulin secretion and proliferation of pancreatic β-cells". Journal of Diabetes. 6 (5): 394–402. doi:10.1111/1753-0407.12161. PMID 24725840. S2CID 13300428.
- ^ Deacon CF (2004). "Circulation and degradation of GIP and GLP-1". Hormone and Metabolic Research. 36 (11–12): 761–5. doi:10.1055/s-2004-826160. PMID 15655705. S2CID 24730915.
- ^ "Diabetes and Intestinal Incretin Hormones: A New Therapeutic Paradigm" at medscape.com (slide 36)
- ^ Toft-Nielsen MB, Madsbad S, Holst JJ (August 2001). "Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes". The Journal of Clinical Endocrinology and Metabolism. 86 (8): 3853–60. doi:10.1210/jcem.86.8.7743. PMID 11502823.
- ^ Meier JJ, Weyhe D, Michaely M, Senkal M, Zumtobel V, Nauck MA, Holst JJ, Schmidt WE, Gallwitz B (March 2004). "Intravenous glucagon-like peptide 1 normalizes blood glucose after major surgery in patients with type 2 diabetes". Critical Care Medicine. 32 (3): 848–51. doi:10.1097/01.CCM.0000114811.60629.B5. PMID 15090972. S2CID 2382791.
- ^ Graaf C, Donnelly D, Wootten D, Lau J, Sexton PM, Miller LJ, Ahn JM, Liao J, Fletcher MM, Yang D, Brown AJ, Zhou C, Deng J, Wang MW (October 2016). "Glucagon-Like Peptide-1 and Its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes". Pharmacological Reviews. 68 (4): 954–1013. doi:10.1124/pr.115.011395. PMC 5050443. PMID 27630114.
- ^ Calanna S, Christensen M, Holst JJ, Laferrère B, Gluud LL, Vilsbøll T, Knop FK (May 2013). "Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies". Diabetologia. 56 (5): 965–72. doi:10.1007/s00125-013-2841-0. PMC 3687347. PMID 23377698.
- ^ a b Rondas D, D'Hertog W, Overbergh L, Mathieu C (September 2013). "Glucagon-like peptide-1: modulator of β-cell dysfunction and death". Diabetes, Obesity & Metabolism. 15 Suppl 3 (3): 185–92. doi:10.1111/dom.12165. PMID 24003936.
- ^ a b Rachmany L, Tweedie D, Li Y, Rubovitch V, Holloway HW, Miller J, Hoffer BJ, Greig NH, Pick CG (October 2013). "Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice". Age. 35 (5): 1621–36. doi:10.1007/s11357-012-9464-0. PMC 3776106. PMID 22892942.
- ^ a b Gautier JF, Choukem SP, Girard J (2008). "Physiology of incretins (GIP and GLP-1) and abnormalities in type 2 diabetes". Diabetes & Metabolism. 34: S65–S72. doi:10.1016/S1262-3636(08)73397-4. PMID 18640588.
- ^ a b Seino Y, Fukushima M, Yabe D (April 2010). "GIP and GLP-1, the two incretin hormones: Similarities and differences". Journal of Diabetes Investigation. 1 (1–2): 8–23. doi:10.1111/j.2040-1124.2010.00022.x. PMC 4020673. PMID 24843404.
- ^ Li H, Lee CH, Yoo KY, Choi JH, Park OK, Yan BC, Byun K, Lee B, Hwang IK, Won MH (December 2010). "Chronic treatment of exendin-4 affects cell proliferation and neuroblast differentiation in the adult mouse hippocampal dentate gyrus". Neuroscience Letters. 486 (1): 38–42. doi:10.1016/j.neulet.2010.09.040. PMID 20854877. S2CID 33327108.
- ^ a b Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Rönnholm H, Wikström L (February 2008). "Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease". Journal of Neuroscience Research. 86 (2): 326–38. doi:10.1002/jnr.21483. PMID 17803225. S2CID 40330443.
- ^ a b c DellaValle B, Hempel C, Johansen FF, Kurtzhals JA (September 2014). "GLP-1 improves neuropathology after murine cold lesion brain trauma". Annals of Clinical and Translational Neurology. 1 (9): 721–32. doi:10.1002/acn3.99. PMC 4241798. PMID 25493285.
- ^ Wang MD, Huang Y, Zhang GP, Mao L, Xia YP, Mei YW, Hu B (December 2012). "Exendin-4 improved rat cortical neuron survival under oxygen/glucose deprivation through PKA pathway". Neuroscience. 226: 388–96. doi:10.1016/j.neuroscience.2012.09.025. PMID 23000625. S2CID 36908739.
- ^ Sharma MK, Jalewa J, Hölscher C (February 2014). "Neuroprotective and anti-apoptotic effects of liraglutide on SH-SY5Y cells exposed to methylglyoxal stress". Journal of Neurochemistry. 128 (3): 459–71. doi:10.1111/jnc.12469. PMID 24112036.
- ^ Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH (September 2002). "Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4". The Journal of Pharmacology and Experimental Therapeutics. 302 (3): 881–8. doi:10.1124/jpet.102.037481. PMID 12183643. S2CID 43716740.
- ^ Iwai T, Sawabe T, Tanimitsu K, Suzuki M, Sasaki-Hamada S, Oka J (April 2014). "Glucagon-like peptide-1 protects synaptic and learning functions from neuroinflammation in rodents". Journal of Neuroscience Research. 92 (4): 446–54. doi:10.1002/jnr.23335. PMID 24464856. S2CID 206129862.
- ^ a b Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH (January 2009). "GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism". Proceedings of the National Academy of Sciences of the United States of America. 106 (4): 1285–90. Bibcode:2009PNAS..106.1285L. doi:10.1073/pnas.0806720106. PMC 2633544. PMID 19164583.
- ^ Wang XH, Li L, Hölscher C, Pan YF, Chen XR, Qi JS (November 2010). "Val8-glucagon-like peptide-1 protects against Aβ1-40-induced impairment of hippocampal late-phase long-term potentiation and spatial learning in rats". Neuroscience. 170 (4): 1239–48. doi:10.1016/j.neuroscience.2010.08.028. PMID 20727946. S2CID 44318394.
- ^ Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, Greig NH (June 2003). "Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron". Journal of Neuroscience Research. 72 (5): 603–12. doi:10.1002/jnr.10611. PMID 12749025. S2CID 83852654.
- ^ DellaValle B, Brix GS, Brock B, Gejl M, Landau AM, Møller A, Rungby J, Larsen A (2016). "Glucagon-Like Peptide-1 Analog, Liraglutide, Delays Onset of Experimental Autoimmune Encephalitis in Lewis Rats". Frontiers in Pharmacology. 7: 433. doi:10.3389/fphar.2016.00433. PMC 5114298. PMID 27917122.
- ^ a b c d Molteni M, Chen E (September 30, 2023). "GLP-1 drugs are transforming diabetes, obesity and more. Could a Nobel be next?". STAT News. Retrieved October 16, 2024.
- ^ Her work paved the way for blockbuster obesity drugs. Now, she's fighting for recognition (Report). 2023-09-08. doi:10.1126/science.adk7627.
- ^ Mojsov S (1992). "Structural requirements for biological activity of glucagon-like peptide-I". International Journal of Peptide and Protein Research. 40 (3–4): 333–343. doi:10.1111/j.1399-3011.1992.tb00309.x. ISSN 0367-8377. PMID 1478791.
- ^ Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF (September 1986). "Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing". Journal of Biological Chemistry. 261 (25): 11880–11889. doi:10.1016/s0021-9258(18)67324-7. ISSN 0021-9258. PMID 3528148.
- ^ Mojsov S, Weir GC, Habener JF (1987-02-01). "Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas". Journal of Clinical Investigation. 79 (2): 616–619. doi:10.1172/JCI112855. ISSN 0021-9738. PMC 424143. PMID 3543057.
- ^ Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF (May 1987). "Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line". Proceedings of the National Academy of Sciences of the United States of America. 84 (10): 3434–3438. Bibcode:1987PNAS...84.3434D. doi:10.1073/pnas.84.10.3434. ISSN 0027-8424. PMC 304885. PMID 3033647.
- ^ O'Rahilly S (2021-04-15). "The islet's bridesmaid becomes the bride: Proglucagon-derived peptides deliver transformative therapies". Cell. 184 (8): 1945–1948. doi:10.1016/j.cell.2021.03.019. ISSN 0092-8674. PMID 33831374. S2CID 233131461.
- ^ Her work paved the way for blockbuster obesity drugs. Now, she's fighting for recognition (Report). 2023-09-08. doi:10.1126/science.adk7627.
- ^ a b Winkler R, Cohen B (June 23, 2023). "Monster Diet Drugs Like Ozempic Started With Actual Monsters". The Wall Street Journal. Retrieved October 16, 2024.
- ^ a b Dunaief D (August 31, 2023). "Setauket scientist Andrew Young's work paves way for drugs like Ozempic". TBR News Media. Retrieved October 16, 2024.
External links
edit- Banting and Best Diabetes Centre at UT glp1
- Glucagon-Like+Peptide+1 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Insulin release pathways
American diabetes association:link-http://diabetes.diabetesjournals.org/content/56/1/8.full