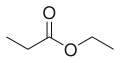
Ethyl propionate is an organic compound with formula C2H5O2CCH2CH3. It is the ethyl ester of propionic acid. It is a colorless volatile liquid with a pineapple-like odor.[3] Some fruits such as kiwis[4] and strawberries[5] contain ethyl propionate in small amounts.
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Ethyl propanoate | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 506287 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.993 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | N119 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H10O2 | |||
| Molar mass | 102.133 g·mol−1 | ||
| Appearance | Colorless Liquid | ||
| Density | 0.884325 g/cm3 | ||
| Melting point | −73.6 °C (−100.5 °F; 199.6 K) | ||
| Boiling point | 98.9 °C (210.0 °F; 372.0 K) | ||
| -66.5·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling:[2] | |||

| |||
| Danger | |||
| H225 | |||
| P403+P235 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 12 °C (54 °F; 285 K) | ||
| 440 °C (824 °F; 713 K) | |||
| Explosive limits | 1.9-11 % | ||
| Safety data sheet (SDS) | [1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Uses and reactions
editIt is also used in the production of some antimalarial drugs including pyrimethamine.[6]
Ethyl propionate can be synthesized by the Fischer esterification of ethanol and propionic acid:
- CH3CH2OH + CH3CH2CO2H → CH3CH2O2CCH2CH3 + H2O
It participates in condensation reactions by virtue of the weakly acidic methylene group.[7]
See also
edit- Methyl propionate, a similar compound
References
edit- ^ "Material Safety Data Sheet : Ethyl propionate" (PDF). Chemblink.com. Archived from the original (PDF) on 2014-12-05. Retrieved 2015-02-27.
- ^ GHS: Record of Ethyl propionate in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ "Ethyl Propionate | Cameo Chemicals | Noaa". Cameochemicals.noaa.gov. Retrieved 2015-02-27.
- ^ Bartley, J. P.; Schwede, A. M. (1989). "Production of volatile compounds in ripening kiwi fruit (Actinidia chinensis)". Journal of Agricultural and Food Chemistry. 37 (4): 1023. doi:10.1021/jf00088a046.
- ^ Perez, A. G.; Rios, J. J.; Sanz, C.; Olias, J. M. (1992). "Aroma components and free amino acids in strawberry variety Chandler during ripening". Journal of Agricultural and Food Chemistry. 40 (11): 2232. doi:10.1021/jf00023a036.
- ^ MacDonald, Thomas (29 January 2016). "Pyrimethamine synthesis: Status at end of 2015". Daraprim Synthesis. Open Source Malaria. Archived from the original on 26 April 2018.
- ^ Cox, Richard F. B.; McElvain, S. M. (1937). "Ethyl Ethoxalylpropionate". Organic Syntheses. 17: 54. doi:10.15227/orgsyn.017.0054.