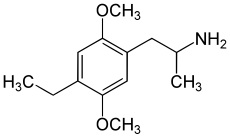
2,5-Dimethoxy-4-ethylamphetamine (DOET, DOE, Hecate) is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin, and was described in his book PiHKAL (Phenethylamines i Have Known And Loved).[2]
 | |
| Clinical data | |
|---|---|
| Other names | 2,5-dimethoxy-4-ethylamphetamine |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H21NO2 |
| Molar mass | 223.316 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Chemistry
editDOET is in a class of compounds commonly known as substituted amphetamines; its full chemical name is 4-ethyl-2,5-dimethoxy-alpha-methylbenzeneethanamine, or 1-(2,5-dimethoxy-4-ethylphenyl)propan-2-amine. It has an active stereocenter and (R)-DOET is the more active enantiomer. DOET is an extremely rare compound and reports of its effects and toxicology in humans are sparse. However, like the more common 2,5-dimethoxy-amphetamine analogues DOB, DOI and DOM, it is a potent and long-acting psychedelic. Removal of the alpha-methyl moiety yields the 2-carbon analogue, commonly known as 2C-E, another psychedelic compound first synthesized by Dr. Alexander Shulgin.
Pharmacology
editSimilarly to related drugs like DOM, DOET likely acts as a 5-HT2A, 5-HT2B, and 5-HT2C receptor partial agonist.[citation needed] It is an agonist of human TAAR1.[3][4]
Effects
editDOET produces psychedelic effects that last up 14–20 hours. In PiHKAL, Shulgin lists the dosage of DOET as being 2–7 mg orally, with 6–7 mg being the dosage for full, desired effects.[2]
Legal status
editInternationally, DOET is a Schedule I controlled drug; under the Convention on Psychotropic Substances, it's legal only for medical uses or scientific research:[1].
United States
editDOET is classified as a Schedule I substance in the United States and is similarly controlled in other parts of the world.
Australia
editDOET is considered a Schedule 9 prohibited substance in Australia under the Poisons Standard (October 2015).[5] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[5]
See also
editReferences
edit- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ a b Shulgin A, Shulgin A (September 1991). PiHKAL: A Chemical Love Story. United States: Transform Press. p. 978. ISBN 0-9630096-0-5.
- ^ Lewin AH, Miller GM, Gilmour B (December 2011). "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorganic & Medicinal Chemistry. 19 (23): 7044–7048. doi:10.1016/j.bmc.2011.10.007. PMC 3236098. PMID 22037049.
- ^ "Compound ID: CHEMBL2360469". ChEMBL. Retrieved 29 April 2014.
- ^ a b "Poisons Standard". Therapeutics Goods Administration. Australian Government. October 2015.