Carbocisteine, also called carbocysteine, is a mucolytic that reduces the viscosity of sputum and so can be used to help relieve the symptoms of chronic obstructive pulmonary disorder (COPD) and bronchiectasis by allowing the sufferer to bring up sputum more easily.[2][3] Carbocisteine should not be used with antitussives (cough suppressants) or medicines that dry up bronchial secretions.
| |

| |
| Names | |
|---|---|
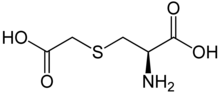
| IUPAC name
(R)-2-Amino-3-(carboxymethylsulfanyl)propanoic acid
| |
| Other names
S-Carboxymethyl-L-cysteine; Actithiol, Lisomucil, Muciclar, Mucodyne, Mucolex, Rhinathiol, Transbronchin[1]
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.010.298 |
| KEGG | |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H9NO4S | |
| Molar mass | 179.19 g·mol−1 |
| Appearance | Colorless solid |
| Melting point | 204 to 207 °C (399 to 405 °F; 477 to 480 K) |
| Pharmacology | |
| R05CB03 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
It was first described in 1951 and came into medical use in 1960.[4] Carbocisteine is produced by alkylation of cysteine with chloroacetic acid.[5]
References
edit- ^ Carbocisteine. drugbank.ca
- ^ Zheng JP, Kang J, Huang SG, Chen P, Yao WZ, Yang L, Bai CX, Wang CZ, Wang C, Chen BY, Shi Y, Liu CT, Chen P, Li Q, Wang ZS, Huang YJ, Luo ZY, Chen FP, Yuan JZ, Yuan BT, Qian HP, Zhi RC, Zhong NS (2008). "Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): A randomised placebo-controlled study". Lancet. 371 (9629): 2013–2018. doi:10.1016/S0140-6736(08)60869-7. PMID 18555912. S2CID 43278410.
- ^ Yasuda H, Yamaya M, Sasaki T, Inoue D, Nakayama K, Tomita N, Yoshida M, Sasaki H (2006). "Carbocisteine reduces frequency of common colds and exacerbations in patients with chronic obstructive pulmonary disease". Journal of the American Geriatrics Society. 54 (2): 378–380. doi:10.1111/j.1532-5415.2005.00592_9.x. PMID 16460403. S2CID 1580116.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 544. ISBN 9783527607495.
- ^ Karlheinz Drauz; Ian Grayson; Axel Kleemann; Hans‐Peter Krimmer; Wolfgang Leuchtenberger; Christoph Weckbecker (2007). "Amino Acids". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_057.pub2. ISBN 978-3527306732.