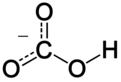
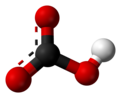
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate[2]) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula HCO−
3.
| |
| |
| Names | |
|---|---|
| IUPAC name
Hydrogencarbonate
| |
| Systematic IUPAC name
Hydroxidodioxidocarbonate(1−)[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 3903504 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| 49249 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HCO− 3 | |
| Molar mass | 61.0168 g mol−1 |
| log P | −0.82 |
| Acidity (pKa) | 10.3 |
| Basicity (pKb) | 7.7 |
| Conjugate acid | Carbonic acid |
| Conjugate base | Carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bicarbonate serves a crucial biochemical role in the physiological pH buffering system.[3]
The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston.[4][5] The name lives on as a trivial name.
Chemical properties
editThe bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula HCO−
3 and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid HNO
3. The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. It is both the conjugate base of carbonic acid H
2CO
3; and the conjugate acid of CO2−
3, the carbonate ion, as shown by these equilibrium reactions:
- CO2−
3 + 2 H2O ⇌ HCO−
3 + H2O + OH− ⇌ H2CO3 + 2 OH−
- H2CO3 + 2 H2O ⇌ HCO−
3 + H3O+ + H2O ⇌ CO2−
3 + 2 H3O+.
A bicarbonate salt forms when a positively charged ion attaches to the negatively charged oxygen atoms of the ion, forming an ionic compound. Many bicarbonates are soluble in water at standard temperature and pressure; in particular, sodium bicarbonate contributes to total dissolved solids, a common parameter for assessing water quality.[6]
Physiological role
editBicarbonate (HCO−
3) is a vital component of the pH buffering system[3] of the human body (maintaining acid–base homeostasis). 70%–75% of CO2 in the body is converted into carbonic acid (H2CO3), which is the conjugate acid of HCO−
3 and can quickly turn into it.[citation needed]
With carbonic acid as the central intermediate species, bicarbonate – in conjunction with water, hydrogen ions, and carbon dioxide – forms this buffering system, which is maintained at the volatile equilibrium[3] required to provide prompt resistance to pH changes in both the acidic and basic directions. This is especially important for protecting tissues of the central nervous system, where pH changes too far outside of the normal range in either direction could prove disastrous (see acidosis or alkalosis). Recently it has been also demonstrated that cellular bicarbonate metabolism can be regulated by mTORC1 signaling.[7]
Additionally, bicarbonate plays a key role in the digestive system. It raises the internal pH of the stomach, after highly acidic digestive juices have finished in their digestion of food. Bicarbonate also acts to regulate pH in the small intestine. It is released from the pancreas in response to the hormone secretin to neutralize the acidic chyme entering the duodenum from the stomach.[8]
Bicarbonate in the environment
editBicarbonate is the dominant form of dissolved inorganic carbon in sea water,[9] and in most fresh waters. As such it is an important sink in the carbon cycle.
Some plants like Chara utilize carbonate and produce calcium carbonate (CaCO3) as result of biological metabolism.[10]
In freshwater ecology, strong photosynthetic activity by freshwater plants in daylight releases gaseous oxygen into the water and at the same time produces bicarbonate ions. These shift the pH upward until in certain circumstances the degree of alkalinity can become toxic to some organisms or can make other chemical constituents such as ammonia toxic. In darkness, when no photosynthesis occurs, respiration processes release carbon dioxide, and no new bicarbonate ions are produced, resulting in a rapid fall in pH.[citation needed]
The flow of bicarbonate ions from rocks weathered by the carbonic acid in rainwater is an important part of the carbon cycle.
Other uses
editThe most common salt of the bicarbonate ion is sodium bicarbonate, NaHCO3, which is commonly known as baking soda. When heated or exposed to an acid such as acetic acid (vinegar), sodium bicarbonate releases carbon dioxide. This is used as a leavening agent in baking.[11]
Ammonium bicarbonate is used in the manufacturing of some cookies, crackers, and biscuits.[12]
Diagnostics
editIn diagnostic medicine, the blood value of bicarbonate is one of several indicators of the state of acid–base physiology in the body. It is measured, along with chloride, potassium, and sodium, to assess electrolyte levels in an electrolyte panel test (which has Current Procedural Terminology, CPT, code 80051).[citation needed]
The parameter standard bicarbonate concentration (SBCe) is the bicarbonate concentration in the blood at a PaCO2 of 40 mmHg (5.33 kPa), full oxygen saturation and 36 °C.[13]
Bicarbonate compounds
editSee also
editReferences
edit- ^ a b "hydrogencarbonate (CHEBI:17544)". Chemical Entities of Biological Interest (ChEBI). UK: European Institute of Bioinformatics. IUPAC Names. Archived from the original on 7 June 2015.
- ^ Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 (PDF), IUPAC, p. 137
- ^ a b c "Clinical correlates of pH levels: bicarbonate as a buffer". Biology.arizona.edu. October 2006. Archived from the original on 31 May 2015.
- ^ William Hyde Wollaston (1814) "A synoptic scale of chemical equivalents", Philosophical Transactions of the Royal Society, 104: 1-22. On page 11, Wollaston coins the term "bicarbonate": "The next question that occurs relates to the composition of this crystallized carbonate of potash, which I am induced to call bi-carbonate of potash, for the purpose of marking more decidedly the distinction between this salt and that which is commonly called a subcarbonate, and in order to refer at once to the double dose of carbonic acid contained in it."
- ^ "Baking Soda". Newton – Ask a Scientist. Argonne National Laboratory. Archived from the original on 26 February 2015. Retrieved 2 May 2018.
- ^ Geor, Raymond J.; Coenen, Manfred; Harris, Pat (31 January 2013). Equine Applied and Clinical Nutrition: Health, Welfare and Performance. Elsevier Health Sciences. p. 90. ISBN 978-0-7020-5418-1.
The most common indicator of water quality is the concentration of total dissolved solids (TDS)
- ^ Ali E, Liponska A, O'Hara B, Amici D, Torno M, Gao P, Asara J, Yap M-N F, Mendillo M, Ben-Sahra I (June 2022). "The mTORC1-SLC4A7 axis stimulates bicarbonate import to enhance de novo nucleotide synthesis". Molecular Cell. 82 (1): 3284–3298.e7. doi:10.1016/j.molcel.2022.06.008. PMC 9444906. PMID 35772404.
- ^ Berne & Levy, Principles of Physiology
- ^ "The chemistry of ocean acidification : OCB-OA". www.whoi.edu. Woods Hole Oceanographic Institution. 24 September 2012. Archived from the original on 19 May 2017. Retrieved 17 May 2017.
- ^ Pełechaty, Mariusz; Pukacz, Andrzej; Apolinarska, Karina; Pełechata, Aleksandra; Siepak, Marcin (June 2013). Porta, Giovanna Della (ed.). "The significance of Chara vegetation in the precipitation of lacustrine calcium carbonate". Sedimentology. 60 (4): 1017–1035. Bibcode:2013Sedim..60.1017P. doi:10.1111/sed.12020. S2CID 128758128.
- ^ "How Baking Soda Works". Serious Eats. Retrieved 23 November 2024.
- ^ "Ammonium Bicarbonate – Bicarbonate Applications". www.ahperformance.com. Retrieved 23 November 2024.
- ^ Acid Base Balance (page 3) Archived 2002-06-13 at the Wayback Machine
External links
edit- Bicarbonates at the U.S. National Library of Medicine Medical Subject Headings (MeSH)