Gold(III) chloride, traditionally called auric chloride, is an inorganic compound of gold and chlorine with the molecular formula Au2Cl6. The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. It has two forms, the monohydrate (AuCl3·H2O) and the anhydrous form, which are both hygroscopic and light-sensitive solids. This compound is a dimer of AuCl3. This compound has a few uses, such as an oxidizing agent and for catalyzing various organic reactions.

| |
 Ball-and-stick model of AuCl3
| |
 Crystal structure of AuCl3
| |
| Names | |
|---|---|
| IUPAC name
Gold(III) trichloride
| |
| Other names
Auric chloride
Gold trichloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.033.280 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| AuCl3 (exists as Au2Cl6) | |
| Molar mass | 606.6511 g/mol |
| Appearance | Red crystals (anhydrous); golden, yellow crystals (monohydrate)[1] |
| Density | 4.7 g/cm3 |
| Melting point | 160 °C (320 °F; 433 K) (decomposes) |
| 68 g/100 ml (20 °C) | |
| Solubility | soluble in ether and ethanol, slightly soluble in liquid ammonia, insoluble in benzene |
| −112·10−6 cm3/mol | |
| Structure | |
| monoclinic | |
| P21/C | |
a = 6.57 Å, b = 11.04 Å, c = 6.44 Å α = 90°, β = 113.3°, γ = 90°[2]
| |
| Square planar | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−117.6 kJ/mol[3] |
| Hazards[4] | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P305+P351+P338 | |
| Related compounds | |
Other anions
|
Gold(III) fluoride Gold(III) bromide |
Other cations
|
Gold(I) chloride Silver(I) chloride Platinum(II) chloride Mercury(II) chloride |
| Supplementary data page | |
| Gold(III) chloride (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
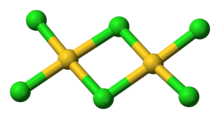
Structure
editAuCl3 exists as a chloride-bridged dimer both as a solid and vapour, at least at low temperatures.[2] Gold(III) bromide behaves analogously.[1] The structure is similar to that of iodine(III) chloride.
Each gold center is square planar in gold(III) chloride, which is typical of a metal complex with a d8 electron count. The bonding in AuCl3 is considered somewhat covalent.[1]
Properties
editGold(III) chloride is a diamagnetic light-sensitive red crystalline solid that forms the orange monohydrate, AuCl3 · H2O; the anhydrous and monohydrate are both hygroscopic. The anhydrous form absorbs moisture from the air to form the monohydrate which can be reversed by the addition of thionyl chloride.[5]
Preparation
editGold(III) chloride was first prepared in 1666 by Robert Boyle by the reaction of metallic gold and chlorine gas at 180 °C:[1][6][7]
- 2 Au + 3 Cl2 → Au2Cl6
This method is the most common method of preparing gold(III) chloride. It can also be prepared by reacting gold powder with iodine monochloride:[5]
- 2 Au + 6 ICl → 2 AuCl3 + 3 I2
The chlorination reaction can be conducted in the presence of tetrabutylammonium chloride, the product being the lipophilic salt tetrabutylammonium tetrachloraurate.[8]
Another method of preparation is via chloroauric acid, which is obtained by first dissolving the gold powder in aqua regia to give chloroauric acid:[9]
- Au + HNO3 + 4 HCl → H[AuCl4] + 2 H2O + NO
The resulting chloroauric acid is subsequently heated in an inert atmosphere at around 100 °C to give Au2Cl6:[10][11]
- 2 H[AuCl4] → Au2Cl6 + 2 HCl
Reactions
editDecomposition
editAnhydrous AuCl3 begins to decompose to AuCl (gold(I) chloride) at around 160 °C (320 °F); however, this, in turn, undergoes disproportionation at higher temperatures to give gold metal and AuCl3:[5][10]
- AuCl3 → AuCl + Cl2 (160 °C)
- 3 AuCl → AuCl3 + 2 Au (>210 °C)
Due to the disproportionation of AuCl, above 210 °C, most of the gold is in the form of elemental gold.[12][11]
Gold(III) chloride is more stable in a chlorine atmosphere and can sublime at around 200 °C without any decomposition. In a chlorine atmosphere, AuCl3 decomposes at 254 °C yielding AuCl which in turn decomposes at 282 °C to elemental gold.[2][13] This fact that no gold chlorides can exist above 400 °C is used in the Miller process.[14]
Other reactions
editAuCl3 is a Lewis acid and readily forms complexes. For example, it reacts with hydrochloric acid to form chloroauric acid (H[AuCl4]):[15]
- HCl + AuCl3 → H+ + [AuCl4]−
Chloroauric acid is the product formed when gold dissolves in aqua regia.[15]
On contact with water, AuCl3 forms acidic hydrates and the conjugate base [AuCl3(OH)]−. A Fe2+ ion may reduce it, causing elemental gold to be precipitated from the solution.[1][16]
Other chloride sources, such as KCl, also convert AuCl3 into [AuCl4]−. Aqueous solutions of AuCl3 react with an aqueous base such as sodium hydroxide to form a precipitate of Au(OH)3, which will dissolve in excess NaOH to form sodium aurate (NaAuO2). If gently heated, Au(OH)3 decomposes to gold(III) oxide, Au2O3, and then to gold metal.[15][17][18][19]
Gold(III) chloride is the starting point for the chemical synthesis of many other gold compounds. For example, the reaction with potassium cyanide produces the water-soluble complex, K[Au(CN)4]:[20]
- AuCl3 + 4 KCN → K[Au(CN)4] + 3 KCl
Gold(III) fluoride can be also produced from gold(III) chloride by reacting it with bromine trifluoride.[15]
Gold(III) chloride reacts with benzene under mild conditions (reaction times of a few minutes at room temperature) to produce the dimeric phenylgold(III) dichloride; a variety of other arenes undergo a similar reaction:[21]
- 2 PhH + Au2Cl6 → [PhAuCl2]2 + 2 HCl
Gold(III) chloride reacts with carbon monoxide in a variety of ways. For example, the reaction of anhydrous AuCl3 and carbon monoxide under SOCl2 produces gold(I,III) chloride with Au(CO)Cl as an intermediate:[22][23]
- 2 AuCl3 + 2 CO → Au4Cl8 + 2 COCl2
If carbon monoxide is in excess, Au(CO)Cl is produced instead.[24][25]
However, under tetrachloroethylene and at 120 °C, gold(III) chloride is first reduced to gold(I) chloride, which further reacts to form Au(CO)Cl. AuCl3 is also known to catalyze the production of phosgene.[25][26]
Applications
editGold(III) chloride has many uses in the laboratory, and primarily thrives in this environment.[5] Regardless, Gold(III) chloride does have a commercial use in red stained glass, as it stabilizes the color.[27]
Organic synthesis
editSince 2003, AuCl3 has attracted the interest of organic chemists as a mild acid catalyst for various reactions,[28] although no transformations have been commercialised. Gold(III) salts, especially Na[AuCl4], provide an alternative to mercury(II) salts as catalysts for reactions involving alkynes. An illustrative reaction is the hydration of terminal alkynes to produce acetyl compounds.[29]
Gold catalyses the alkylation of certain aromatic rings and the conversion of furans to phenols. Some alkynes undergo amination in the presence of gold(III) catalysts. For example, a mixture of acetonitrile and gold(III) chloride catalyses the alkylation of 2-methylfuran by methyl vinyl ketone at the 5-position:[30]
The efficiency of this organogold reaction is noteworthy because both the furan and the ketone are sensitive to side reactions such as polymerisation under acidic conditions. In some cases where alkynes are present, phenols sometimes form (Ts is an abbreviation for tosyl):[30]
This reaction involves a rearrangement that gives a new aromatic ring.[31]
Another example of an AuCl3 catalyzed reaction is a hydroarylation, which is basically a Friedel-Crafts reaction using metal-alkyne complexes. Example, the reaction of mesitylene with phenylacetylene:[32]
Gold(III) chloride can be used for the direct oxidation of primary amines into ketones, such as the oxidation of cyclohexylamine to cyclohexanone.[5]
This reaction is pH sensitive, requiring a mildly acidic pH to proceed, however, it does not require any additional steps.[5]
In the production of organogold(III) compounds, AuCl3 is used as a source of gold. A main example of this is the production of monoarylgold(III) complexes, which are produced by direct electrophilic auration of arenes by gold(III) chloride.[33]
Gold nanoparticles
editGold(III) chloride is used in the synthesis of gold nanoparticles, which are extensively studied for their unique size-dependent properties and applications in fields such as electronics, optics, and biomedicine. Gold nanoparticles can be prepared by reducing gold(III) chloride with a reducing agent such as sodium tetrafluoroborate, followed by stabilization with a capping agent.[34]
Photography
editGold(III) chloride has been used historically in the photography industry as a sensitizer in the production of photographic films and papers. However, with the advent of digital photography, its use in this field has diminished.[35]
Natural occurrence
editThis compound does not occur naturally; however, a similar compound with the formula AuO(OH,Cl)·nH2O is known as a product of natural gold oxidation.[36][37]
References
edit- ^ a b c d e Egon Wiberg; Nils Wiberg; A. F. Holleman (2001). Inorganic Chemistry (101 ed.). Academic Press. pp. 1286–1287. ISBN 978-0-12-352651-9.
- ^ a b c E. S. Clark; D. H. Templeton; C. H. MacGillavry (1958). "The crystal structure of gold(III) chloride". Acta Crystallogr. 11 (4): 284–288. doi:10.1107/S0365110X58000694. Retrieved 2010-05-21.
- ^ Haynes, William M.; Lide, David R.; Bruno, Thomas J., eds. (2016). CRC Handbook of Chemistry and Physics: A Ready-reference Book of Chemical and Physical Data (95th ed.). Boca Raton, Florida. p. 5-5. ISBN 978-1-4987-5428-6. OCLC 930681942.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ "Gold Chloride". American Elements. Retrieved July 22, 2019.
- ^ a b c d e f Michael J. Coghlan; Rene-Viet Nguyen; Chao-Jun Li; Daniel Pflästerer; A. Stephen K. Hashmi (2015). "Gold(III) Chloride". Encyclopedia of Reagents for Organic Synthesis: 1–24. doi:10.1002/047084289X.rn00325.pub3. ISBN 9780470842898.
- ^ Robert Boyle (1666). The origine of formes and qualities. p. 370.
- ^ Thomas Kirke Rose (1895). "The dissociation of chloride of gold". Journal of the Chemical Society, Transactions. 67: 881–904. doi:10.1039/CT8956700881.
- ^ Buckley, Robbie W.; Healy, Peter C.; Loughlin, Wendy A. (1997). "Reduction of [NBu4][AuCl4] to [NBu4][AuCl2] with Sodium Acetylacetonate". Australian Journal of Chemistry. 50 (7): 775. doi:10.1071/C97029.
- ^ Block, B. P. (1953). "Gold Powder and Potassium Tetrabromoaurate(III)". Inorganic Syntheses. Inorganic Syntheses. Vol. 4. pp. 14–17. doi:10.1002/9780470132357.ch4. ISBN 9780470132357.
- ^ a b Ya-jie Zheng; Wei Guo; Meng Bai; Xing-wen Yang (2006). "Preparation of chloroauric acid and its thermal decomposition". The Chinese Journal of Nonferrous Metals (in Chinese). 16 (11): 1976–1982. Archived from the original on March 27, 2024.
- ^ a b Robert G. Palgrave; Ivan P. Parkin (2007). "Aerosol Assisted Chemical Vapor Deposition of Gold and Nanocomposite Thin Films from Hydrogen Tetrachloroaurate(III)". Chemistry of Materials. 19 (19). ACS Publications: 4639–4647. doi:10.1021/cm0629006.
- ^ Yiqin Chen; Xuezeng Tian; Wei Zeng; Xupeng Zhu; Hailong Hu; Huigao Duan (2015). "Vapor-phase preparation of gold nanocrystals by chloroauric acid pyrolysis". Journal of Colloid and Interface Science. 439. Elsevier: 21–27. Bibcode:2015JCIS..439...21C. doi:10.1016/j.jcis.2014.10.017. PMID 25463171.
- ^ E.M.W. Janssen; J.C.W. Folmer; G.A. Wiegers (1974). "The preparation and crystal structure of gold monochloride, AuCl". Journal of the Less Common Metals. 38 (1): 71–76. doi:10.1016/0022-5088(74)90204-5.
- ^ Hermann Renner; Günther Schlamp (2000). "Gold, Gold Alloys, and Gold Compounds". Ullmann's Encyclopedia of Industrial Chemistry. pp. 106–107. doi:10.1002/14356007.a12_499. ISBN 978-3-527-30673-2.
- ^ a b c d N. N. Greenwood; A. Earnshaw (1997). Chemistry of the Elements (2 ed.). Oxford, UK: Butterworth-Heinemann. pp. 1184–1185. ISBN 9780750633659.
- ^ Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry; John Wiley & Sons: New York, 1999; pp. 1101-1102
- ^ The Merck Index. An Encyclopaedia of Chemicals, Drugs and Biologicals. 14. Ed., 2006, p. 780, ISBN 978-0-911910-00-1.
- ^ H. Nechamkin, The Chemistry of the Elements, McGraw-Hill, New York, 1968, p. 222
- ^ A. F. Wells, Structural Inorganic Chemistry, 5th ed., Oxford University Press, Oxford, UK, 1984, p. 909
- ^ Henry K. Lutz (1961). "Synthesis and Analyses of KAu(CN)4". Honors Theses. Union Digital Works.
- ^ Li, Zigang; Brouwer, Chad; He, Chuan (2008-08-01). "Gold-Catalyzed Organic Transformations". Chemical Reviews. 108 (8): 3239–3265. doi:10.1021/cr068434l. ISSN 0009-2665. PMID 18613729.
- ^ Daniela Belli Dell'Amico; Fausto Calderazzo; Fabio Marchetti; Stefano Merlino; Giovanni Perego (1977). "X-Ray crystal and molecular structure of Au4Cl8, the product of the reduction of Au2Cl6 by Au(CO)Cl". Journal of the Chemical Society, Chemical Communications: 31–32. doi:10.1039/C39770000031.
- ^ Daniela Belli Dell'Amico; Fausto Calderazzo; Fabio Marchetti; Stefano Merlino (1982). "Synthesis and molecular structure of [Au4Cl8], and the isolation of [Pt(CO)Cl5]– in thionyl chloride". Journal of the Chemical Society, Dalton Transactions (11): 2257–2260. doi:10.1039/DT9820002257.
- ^ Dell'Amico, D. Belli; Calderazzo, F.; Murray, H. H.; Fackler, J. P. (1986). "Carbonylchlorogold(I)". Inorganic Syntheses. Vol. 24. pp. 236–238. doi:10.1002/9780470132555.ch66. ISBN 9780470132555.
- ^ a b T.A. Ryan; E.A. Seddon; K.R. Seddon; C. Ryan (1996). Phosgene And Related Carbonyl Halides. Elsevier Science. pp. 242–243. ISBN 9780080538808.
- ^ M. S. Kharasch; H. S. Isbell (1930). "The Chemistry of Organic Gold Compounds. I. Aurous Chloride Carbonyl and a Method of Linking Carbon to Carbon". Journal of the American Chemical Society. 52 (7): 2919–2927. doi:10.1021/ja01370a052.
- ^ "Stained glass: art & function | HWNY". Historical Windows of New York. 2020-07-29. Retrieved 2024-10-29.
- ^ G. Dyker, An Eldorado for Homogeneous Catalysis?, in Organic Synthesis Highlights V, H.-G. Schmaltz, T. Wirth (eds.), pp 48–55, Wiley-VCH, Weinheim, 2003
- ^ Y. Fukuda; K. Utimoto (1991). "Effective transformation of unactivated alkynes into ketones or acetals with a gold(III) catalyst". J. Org. Chem. 56 (11): 3729. doi:10.1021/jo00011a058.
- ^ a b A. S. K. Hashmi; T. M. Frost; J. W. Bats (2000). "Highly Selective Gold-Catalyzed Arene Synthesis". J. Am. Chem. Soc. 122 (46): 11553. doi:10.1021/ja005570d.
- ^ A. Stephen; K. Hashmi; M. Rudolph; J. P. Weyrauch; M. Wölfle; W. Frey; J. W. Bats (2005). "Gold Catalysis: Proof of Arene Oxides as Intermediates in the Phenol Synthesis". Angewandte Chemie International Edition. 44 (18): 2798–801. doi:10.1002/anie.200462672. PMID 15806608.
- ^ Reetz, M. T.; Sommer, K. (2003). "Gold-Catalyzed Hydroarylation of Alkynes". European Journal of Organic Chemistry. 2003 (18): 3485–3496. doi:10.1002/ejoc.200300260.
- ^ Kharasch, M. S.; Isbell, Horace S. (1931-08-01). "The Chemistry of Organic Gold Compounds. III. Direct Introduction of Gold into the Aromatic Nucleus (Preliminary Communication)". Journal of the American Chemical Society. 53 (8): 3053–3059. doi:10.1021/ja01359a030. ISSN 0002-7863.
- ^ M. Lin; C. M. Sorensen; K. J. Klabunde (1999). "Ligand-Induced Gold Nanocrystal Superlattice Formation in Colloidal Solution". Chemistry of Materials. 11 (2): 198–202. doi:10.1021/cm980665o.
- ^ Philip Ellis (1975). "Gold in photography". Gold Bulletin. 8: 7–12. doi:10.1007/BF03215055. S2CID 136538890.
- ^ "UM1995-16-O:AuClH". mindat.org. Retrieved 27 April 2023.
- ^ John L. Jambor; Nikolai N. Pertsev; Andrew C. Roberts (1996). "New Mineral Names" (PDF). American Mineralogist. 81: 768.
External links
edit- Media related to Gold trichloride at Wikimedia Commons