Indoxacarb is an oxadiazine pesticide developed by DuPont that acts against lepidopteran larvae. It is marketed under the names Indoxacarb Technical Insecticide, Steward Insecticide and Avaunt Insecticide. It is also used as the active ingredient in the Syngenta line of commercial pesticides: Advion and Arilon.[1][2][3]
| |
| Names | |
|---|---|
| Preferred IUPAC name
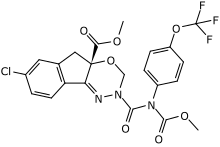
Methyl 7-chloro-2,5-dihydro-2-[[(methoxycarbonyl)[4-(trifluoromethoxy)phenyl]amino]carbonyl]indeno[1,2-e][1,3,4]oxadiazine-4a(3H)-carboxylate | |
| Systematic IUPAC name
(S)-Methyl 7-chloro-2-{[(methoxycarbonyl)[4-(trifluoromethoxy)phenyl]amino]carbonyl}-2H,3H,4aH,5H-indeno[1,2-e][1,3,4]oxadiazine-4a-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | DPX-MP062 |
| 8366683 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.132.370 |
| KEGG | |
| MeSH | Indoxacarb |
PubChem CID
|
|
| UNII | |
| UN number | UN 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C22H17ClF3N3O7 | |
| Molar mass | 527.84 g·mol−1 |
| Melting point | 88.1 °C (190.6 °F; 361.2 K) 99% indoxacarb PAI |
| Pharmacology | |
| QP53AX27 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Its main mode of action is via blocking of neuronal sodium channels. It is fairly lipophilic with a Kow of 4.65. This pesticide should be used with caution since some insects such as the oriental tobacco budworm (Helicoverpa assulta) become resistant when exposed.[4]
In 2021, the European Union[5] chose not to renew Indoxacarb for use as a plant-protection insecticide. The United Kingdom still allows use of the compound until 2025.[6]
Development
editIndoxacarb was developed by the McCann et al. team at E. I. du Pont de Nemours.[7][8]
Household products
editIndoxacarb is the active ingredient in a number of household insecticides, including cockroach and ant baits, and can remain active after digestion.[9] In 2012 DuPont's Professional Products including the line of Advion and Arilon products was purchased by Syngenta.[10] Indoxacarb is the active ingredient in the pet product, Activyl, from Merck Animal Health. It is marketed to kill fleas on dogs and cats.[11]
Toxicity to humans
editWhile toxicity to humans has not been formally studied, there is a reported case of a person consuming indoxacarb in a suicide attempt.[12] The patient developed methemoglobinemia following ingestion.[12] Methemoglobinemia (also known as blue baby syndrome) is a condition which ultimately decreases the effectiveness of red blood cells to exchange oxygen with organs. Methemoglobinemia can be fatal if left untreated, however when the cause is exposure to a chemical agent (not genetic) a variety of treatments are available and effective.[13][14]
References
edit- ^ United States Environmental Protection Agency. Office of Prevention, Pesticides and Toxic Substances (7505C). Pesticide Fact Sheet. Name of Chemical: Indoxacarb. Reason for Issuance: Conditional Registration. Date Issued: October 30, 2000. Archived May 2, 2004, at the Wayback Machine
- ^ United States Environmental Protection Agency. Federal Register: Indoxacarb; Pesticide Tolerance. Federal Register: July 11, 2007 (Volume 72, Number 132)
- ^ Commission Directive 2006/10/EC of 27 January 2006 amending Council Directive 91/414/EEC to include forchlorfenuron and indoxacarb as active substances. Official Journal of the European Union 2006-1-28
- ^ Wang, Kai-Yun; Zhang, Yong; Wang, Hong-Yan; Xia, Xiao-Ming; Liu, Tong-Xian (2010-01-01). "Influence of three diets on susceptibility of selected insecticides and activities of detoxification esterases of Helicoverpa assulta (Lepidoptera: Noctuidae)". Pesticide Biochemistry and Physiology. 96 (1): 51–55. doi:10.1016/j.pestbp.2009.09.003.
- ^ "C/2021/8467, Commission Implementing Regulation (EU) 2021/2081 of 26 November 2021 concerning the non-renewal of approval of the active substance indoxacarb, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending Commission Implementing Regulation (EU) No 540/2011 (Text with EEA relevance)". 26 November 2021.
- ^ "UK authorised biocidal products - Biocides - HSE".
- ^ McCann, Stephen F; Annis, Gary D; Shapiro, Rafael; Piotrowski, David W; Lahm, George P; Long, Jeffery K; Lee, Kevin C; Hughes, Margaret M; Myers, Brian J; Griswold, Sandra M; Reeves, Bonita M; March, Robert W; Sharpe, Paula L; Lowder, Patrick; Barnette, William E; Wing, Keith D (2001). "The discovery of indoxacarb: oxadiazines as a new class of pyrazoline-type insecticides". Pest Management Science. 57 (2). Society of Chemical Industry (Wiley): 153–164. doi:10.1002/1526-4998(200102)57:2<153::aid-ps288>3.0.co;2-o. ISSN 1526-498X. PMID 11455646.
- ^ McCann, Stephen F.; Annis, Gary D.; Shapiro, Rafael; Piotrowski, David W.; Lahm, George P.; Long, Jeffrey K.; Lee, Kevin C.; Hughes, Margaret M.; Myers, Brian J.; Griswold, Sandra M.; Reeves, Bonita M.; March, Robert W.; Sharpe, Paula L.; Lowder, Patrick; Tseng, Paul; Barnette, William E.; Wing, Keith D. (2001-07-23). "Synthesis and Biological Activity of Oxadiazine and Triazine Insecticides: The Discovery of Indoxacarb". Synthesis and Chemistry of Agrochemicals VI. ACS Symposium Series. Vol. 800. Washington, DC: American Chemical Society. pp. 166–177. doi:10.1021/bk-2002-0800.ch016. ISBN 9780841237834. ISSN 0097-6156.
- ^ "Indoxacarb Insecticide Wipes Out Entire Cockroach Generations". June 23, 2008. Archived from the original on June 27, 2008. Retrieved 2009-12-14.
- ^ "Syngenta Acquires DuPont Professional Products Insecticide Business". syngenta-us.com.
- ^ "Activyl". merck-animal-health-usa.com.
- ^ a b Prasanna, Lakshmi; Rao, S. Manimala; Singh, Vishal; Kujur, Rash; Gowrishankar (2008). "Indoxacarb poisoning: An unusual presentation as methemoglobinemia". Indian Journal of Critical Care Medicine. 12 (4): 198–200. doi:10.4103/0972-5229.45082. ISSN 0972-5229. PMC 2738321. PMID 19742262.
- ^ Rehman, Habib Ur (September 2001). "Methemoglobinemia". The Western Journal of Medicine. 175 (3): 193–196. doi:10.1136/ewjm.175.3.193. PMC 1071541. PMID 11527852.
- ^ Ludlow, John T.; Wilkerson, Richard G.; Nappe, Thomas M. (2023), "Methemoglobinemia", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30726002, retrieved 2023-09-01
Further reading
edit- Lapied, Bruno; Françoise Grolleau; David B Sattelle (January 2001). "Indoxacarb, an oxadiazine insecticide, blocks insect neuronal sodium channels". Br J Pharmacol. 132 (2): 587–595. doi:10.1038/sj.bjp.0703853. PMC 1572588. PMID 11159709.
- Khambay, Bhupinder P.S. (2002). "Pyrethroid Insecticides". Pesticide Outlook. 13 (2): 49–54. doi:10.1039/b202996k.
- Moncada, Adriana. Environmental Fate of Indoxacarb. Environmental Monitoring Branch, Department of Pesticide Regulation, State of California. March 6, 2003
- Tillman, P Glynn; Hammes, Glenn G; Sacher, Matthew; Connair, Michael; Brady, E Angela; Wing, Keith D (January 2002). "Toxicity of a formulation of the insecticide indoxacarb to the tarnished plant bug, Lygus lineolaris (Hemiptera: Miridae), and the big-eyed bug, Geocoris punctipes (Hemiptera: Lygaeidae)". Pest Manag. Sci. 58 (1): 92–100. doi:10.1002/ps.426. PMID 11838290. Archived from the original on 2008-08-07.
External links
edit- DuPont Steward insecticide - FAQs. Updated 20 January 2007. Archived 21 August 2008 at the Wayback Machine Retrieved 2012-11-11
- Indoxacarb in the Pesticide Properties DataBase (PPDB)