5α-Dihydronormethandrone (5α-DHNMT; developmental code name RU-575), also known as 17α-methyl-4,5α-dihydro-19-nortestosterone or as 17α-methyl-5α-estran-17β-ol-3-one, is an androgen/anabolic steroid and a likely metabolite of normethandrone formed by 5α-reductase.[1][2] Analogously to nandrolone and its 5α-reduced metabolite 5α-dihydronandrolone, 5α-DHNMT shows reduced affinity for the androgen receptor relative to normethandrone.[1] Its affinity for the androgen receptor is specifically about 33 to 60% of that of normethandrone.[1]
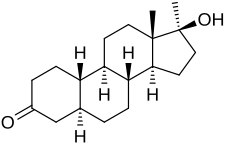 | |
| Clinical data | |
|---|---|
| Other names | 5α-DHNMT; RU-575; 17α-Methyl-4,5α-dihydro-19-nortestosterone; 17α-Methyl-5α-estran-17β-ol-3-one |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C19H30O2 |
| Molar mass | 290.447 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Normethandrone | 75–125 | 125–150 | <1 | 1–5 | <1 | ? | ? |
| 5α-Dihydronormethandrone | 15–25 | 50–75 | ? | <1 | ? | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, estradiol for the ER, dexamethasone for the GR, and aldosterone for the MR. Sources: See template. | |||||||
See also
editReferences
edit- ^ a b c Ojasoo T, Delettré J, Mornon JP, Turpin-VanDycke C, Raynaud JP (1987). "Towards the mapping of the progesterone and androgen receptors". J. Steroid Biochem. 27 (1–3): 255–69. doi:10.1016/0022-4731(87)90317-7. PMID 3695484.
- ^ Schjølberg, T. H. (2013). In Vitro Synthesis of Metabolites of three Anabolic Androgenic Steroids, by Human Liver Microsomes (Master's thesis, Institutt for bioteknologi). https://brage.bibsys.no/xmlui/handle/11250/246018