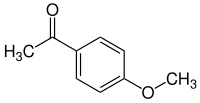
Acetanisole is an aromatic chemical compound with an aroma described as sweet, fruity, nutty, and similar to vanilla. In addition acetanisole can sometimes smell like butter or caramel.[3] Its chemical names are based on considering the structure as either an acetyl (methyl-ketone) analog of anisole. Other names It can also be seen as a methyl ether analog of acetophenone.
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-(4-Methoxyphenyl)ethan-1-one | |
| Other names
4-Acetylanisole; para-Acetanisole; 4-Methoxyacetophenone; Linarodin; Novatone; Vananote; Castoreum anisole; 4-Methoxyphenyl methyl ketone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.002.560 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H10O2 | |
| Molar mass | 150.177 g·mol−1 |
| Appearance | White to pale yellow crystals[1] |
| Density | 1.094 g/cm3 |
| Melting point | 38.2 °C (100.8 °F; 311.3 K)[2] |
| Boiling point | 254 °C (489 °F; 527 K)[2] |
| 2470 mg/L[1] | |
| Hazards | |
| Flash point | 138 °C (280 °F)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Acetanisole is found naturally in castoreum, the glandular secretion of the beaver.[1]
Preparation
editAcetanisole can be prepared synthetically by Friedel-Crafts acylation of anisole with acetyl chloride:
Application
editIt is used as a cigarette additive,[4] a fragrance,[1] and a flavoring in food.[5]
Reactions
edit4-Methoxyacetophenone is a standard substrate or product of much research, such as transfer hydrogenation[6] and directed arylations.[7]
References
edit- ^ a b c d Para-Acetanisole, The Good Scents Company
- ^ a b 4'-Methoxyacetophenone from PubChem
- ^ a b Acetanisole at Sigma-Aldrich
- ^ Tobacco Documents | Profiles | Additives | Acetanisole Archived April 11, 2008, at the Wayback Machine
- ^ 21 CFR 172.515
- ^ Noyori, Ryoji; Yamakawa, Masashi; Hashiguchi, Shohei (2001). "Metal−Ligand Bifunctional Catalysis: A Nonclassical Mechanism for Asymmetric Hydrogen Transfer between Alcohols and Carbonyl Compounds". The Journal of Organic Chemistry. 66 (24): 7931–7944. doi:10.1021/jo010721w. PMID 11722188.
- ^ Palucki, Michael; Buchwald, Stephen L. (1997). "Palladium-Catalyzed α-Arylation of Ketones". Journal of the American Chemical Society. 119 (45): 11108–11109. doi:10.1021/ja972593s.