1-Diazidocarbamoyl-5-azidotetrazole
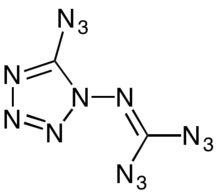
1-Diazidocarbamoyl-5-azidotetrazole, often jokingly referred to as azidoazide azide,[5] is a heterocyclic inorganic compound with the formula C2N14.[6] It is a highly reactive and extremely sensitive explosive.
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-(5-Azido-1H-tetrazol-1-yl)carbonimidoyl diazide | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | AA |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2N14 | |
| Molar mass | 220.120 g·mol−1 |
| Density | 1.723 g·cm−3[2] |
| Melting point | 78 °C (172 °F; 351 K) |
| Boiling point | Violent explosion at 110 °C |
| Solubility | Soluble in diethyl ether, acetone, hydrocarbons, chlorinated hydrocarbons[3] |
| Structure[4] | |
| orthorhombic | |
| Pbcn | |
a = 18.1289, b = 8.2128, c = 11.4021
| |
Lattice volume (V)
|
1697.6 |
Formula units (Z)
|
8 |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
357 kcal·mol−1[5] (1495 kJ·mol−1)[2] |
| Explosive data | |
| Shock sensitivity | <0.25 J |
| Friction sensitivity | <1 N |
| Detonation velocity | 8960 m·s−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
will unpredictably and violently detonate-Part of the nitrogen highly energetic compounds family. |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
edit1-Diazidocarbamoyl-5-azidotetrazole was produced by diazotizing triaminoguanidinium chloride with sodium nitrite in ultra-purified water.[6] Another synthesis uses a metathesis reaction between isocyanogen tetrabromide in acetone and aqueous sodium azide.[3] This first forms isocyanogen tetraazide, the "open" isomer of C2N14, which at room temperature quickly undergoes an irreversible cyclization reaction to form a tetrazole ring.[7]
Properties
editThe C2N14 molecule is a monocyclic tetrazole with three azide groups. This ring form is in equilibrium with isocyanogen tetraazide, an isomeric acyclic structure that has long been known to cyclize quickly to the tetrazole.[7]
It is one of a family of high energy nitrogen compounds in which the nitrogen atoms do not have strong triple bonds. This instability makes many such compounds liable to explosive decomposition, releasing nitrogen gas.
This tetrazole explosive has a decomposition temperature of 124 °C. It is very sensitive, with an impact sensitivity lower than 0.25 joules. It is, however, less sensitive than nitrogen triiodide. Decomposition can be initiated by only using contact or using a laser beam.[8] For these reasons, it is often erroneously claimed to be the world's most sensitive compound.[9][5]
See also
edit- The dictionary definition of azidoazide azide at Wiktionary
References
edit- ^ "Azidoazide azide". American Chemical Society. 17 August 2020. Archived from the original on 28 October 2020. Retrieved 13 September 2021.
- ^ a b Martin, Franz Albert. "Novel Energetic Materials based on 1,5-Diaminotetrazole and 3,5-Diamino-1H-1,2,4-triazole" (PDF). pp. 80–87. Archived (PDF) from the original on 16 April 2021. Retrieved 24 February 2018.
- ^ a b US 2990412, Grundmann, Christoph J. & Schnabel, Wilhelm Joseph, "Isocyanogen tetraazide and its preparation", published 1961-06-27, assigned to Olin Mathieson Chemical Corp.
- ^ Klapötke, Thomas M.; Martin, Franz A.; Stierstorfer, Jörg (26 April 2011). "C2N14: An Energetic and Highly Sensitive Binary Azidotetrazole". Angewandte Chemie International Edition. 50 (18): 4227–4229. doi:10.1002/anie.201100300. PMID 21472944.
- ^ a b c Lowe, Derek (9 January 2013). "Things I Won't Work With: Azidoazide Azides, More Or Less". Science Magazine. American Association for the Advancement of Science. Archived from the original on 7 May 2021. Retrieved 24 February 2018.
- ^ a b Klapötke, Thomas M.; Martin, Franz A.; Stierstorfer, Jörg (26 April 2011). "C2N14: An Energetic and Highly Sensitive Binary Azidotetrazole". Angewandte Chemie International Edition. 50 (18): 4227–4229. doi:10.1002/anie.201100300. PMID 21472944.
- ^ a b Banert, Klaus; Richter, Sebastian; Schaarschmidt, Dieter; Lang, Heinrich (2013). "Well Known or New? Synthesis and Structure Assignment of Binary C2N14 Compounds Reinvestigated". Angewandte Chemie International Edition. 52 (12): 3499–3502. doi:10.1002/anie.201209170. ISSN 1521-3773. PMID 23404921.
- ^ Klapötke, Thomas M.; Krumm, Burkhard; Martin, Franz A.; Stierstorfer, Jörg (2011-11-09). "New Azidotetrazoles: Structurally Interesting and Extremely Sensitive". Chemistry: An Asian Journal. 7 (1): 214–224. doi:10.1002/asia.201100632. ISSN 1861-4728. PMID 22069147. S2CID 27239569.
- ^ "Beware Of Azidoazide Azide, The World's Most Explosive Chemical". Discovery. Retrieved 2022-04-05.