In organic chemistry hydroxyanthraquinones refers to compounds with the formula C12H8-n(OH)n(CO)2 where n ≥ 1. Almost all hydroxyanthraquinones are derivative of 9,10-anthraquinone.[3][4][5]
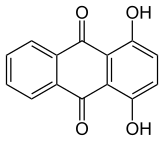
Isomers
editOne peculiarity of the hydroxyanthraquinones is the relative obscurity of the monohydroxy derivatives. Most hydroxyanthraquinones have two or more hydroxy groups.
Dihydroxy
editDihydroxyanthraquinones have the formula C12H6(OH)2(CO)2. The dyes alizarin (1,2-Dihydroxyanthraquinone) and quinizarin are prominent examples.
Trihydroxy
editTrihydroxyanthraquinones have the formula C12H5(OH)3(CO)2. 1,2,4-Trihydroxyanthraquinone, commonly called purpurin, is a naturally occurring red/yellow dye.
Tetrahydroxy
editTetrahydroxyanthraquinones have the formula C12H4(OH)4(CO)2. The dye quinalizarin (1,2,5,8-tetrahydroxyanthraquinone is one example.
Hexahydroxy
editDihydroxyanthraquinones have the formula C12H2(OH)6(CO)2.1,2,3,5,6,7-hexahydroxy-9,10-anthraquinone (rufigallol) occurs in nature.
See also
editReferences
edit- ^ Bien, H.-S.; Stawitz, J.; Wunderlich, K. "Anthraquinone Dyes and Intermediates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_355. ISBN 978-3527306732.
- ^ Bigelow, L. A.; Reynolds, H. H. (1926). "Quinizarin". Org. Synth. 6: 78. doi:10.15227/orgsyn.006.0078.
- ^ Khalafy, J.; Bruce, J. M. (2002). "Oxidative Dehydrogenation of 1-Tetralones: Synthesis of Juglone, Naphthazarin, and α-Hydroxyanthraquinones" (pdf). Journal of Sciences, Islamic Republic of Iran. 13 (2): 131–139.
- ^ Thomson, R. H. (1971). Naturally Occurring Quinones. London: Academic Press. Quoted by Khalafy and Bruce.
- ^ Thomson, R. H. (1987). Naturally Occurring Quinones III. London: Chapman and Hall. Quoted by Khalafy and Bruce.